Abstract
Background. Polyethylene glycols (PEGs) are widely applied in technology of pharmaceutical products, as well as in other branches of industry. Owing to their physicochemical properties, they are particularly effective in enhancing the solubility of a wide range of drug formulations.
Objectives. The aim of the study was to evaluate the effect of PEGs on the physicochemical parameters of model systems containing different concentrations of sodium chloride (NaCl), with particular emphasis on the specific conductivity of these solutions.
Materials and methods. A series of aqueous solutions containing 0.9% and 9.0% NaCl, with increasing mass concentrations (2.0–25.0% w/w) of PEG 200 and PEG 4000, were prepared and analyzed. Their specific conductivity was tested using a CC-505 conductivity meter with an EC-70 conductivity sensor; also, densities and viscosities of the tested solutions were evaluated.
Results. The increased concentration of polymers in the system resulted in decrease of the specific conductivity and molar conductivity in each of the series of evaluated solutions. An inverse relationship occurred in viscosity measurements, which increased with increasing PEG content in the system.
Conclusions. The addition of PEG 200 and PEG 4000 to aqueous NaCl solutions affected both the specific conductivity and viscosity of these systems. Both types of polymers had similar effects on conductivity changes in 0.9% and 9.0% NaCl solutions.
Key words: polyethylene glycols, specific conductivity, molar conductivity, sodium chloride, viscosity
Streszczenie
Wprowadzenie. Glikole polietylenowe (PEG) znajdują szerokie zastosowanie w technologii produktów farmaceutycznych, a także w innych gałęziach przemysłu. Ze względu na swoje właściwości można je stosować w celu zwiększenia rozpuszczalności różnych substancji leczniczych.
Cel pracy. Celem badań była ocena wpływu PEG-ów na parametry fizykochemiczne układów modelowych zawierających różne stężenia chlorku sodu, ze szczególnym uwzględnieniem przewodnictwa właściwego tych roztworów.
Materiał i metody. Badano serię roztworów 0,9% i 9,0% chlorku sodu w wodzie z dodatkiem PEG200 i PEG4000 o rosnących stężeniach masowych (2,0–25,0% w/w). Ich przewodność właściwą badano za pomocą konduktometru CC-505 z czujnikiem przewodności EC-70; oceniano także gęstości i lepkości badanych roztworów.
Wyniki. Zwiększone stężenie polimerów w układzie spowodowało zmniejszenie przewodności właściwej i przewodności molowej w każdej z serii ocenianych roztworów. Odwrotna zależność wystąpiła w pomiarach lepkości, która wzrastała wraz ze wzrostem zawartości glikolu polietylenowego w układzie.
Wnioski. Dodatek PEG200 i PEG4000 do wodnych roztworów chlorku sodu wpływał zarówno na przewodność właściwą, jak i lepkość tych układów. Obydwa typy polimerów miały podobny wpływ na zmiany przewodnictwa w 0,9% i 9,0% roztworach chlorku sodu.
Słowa kluczowe: glikole polietylenowe, przewodność właściwa, przewodność molowa, chlorek sodu, lepkość
Introduction
Synthetic polymers are a very extensive group of chemical compounds that are used on a daily basis in many areas of industry, medicine and pharmacy. Detailed knowledge of the physicochemical properties of available polymers can also contribute to advancements in pharmaceutical technology. One of the frequently used groups of polymers are polyethylene glycols (PEGs). Their physical properties change with increasing molecular weight. These polymers are characterized by an increase in the melting temperature with increasing molecular weight. For example, PEGs with molecular weights between 100 and 700 are liquids, those between 1000 and 2000 are soft solids, and PEGs with molecular weights above 2000 are hard crystalline solids.1 This property enables the formulation of semi-solid drug forms using mixtures of PEGs with different molar masses.2 Owing to their characteristic properties – namely, amphiphilicity and relatively high biocompatibility – PEGs are frequently used in pharmaceutical technology, particularly to enhance the solubility of active substances in dosage forms such as tablets, capsules and ointments.1 PEG 4000 is particularly highlighted here due to its very low toxicity and high biocompatibility; it is currently one of the most commonly used polymers for the synthesis of targeted drug delivery systems.3 Another polymer commonly used in pharmaceutical systems is PEG 200, which facilitates the formulation of homogeneous liquid preparations containing hydrophobic drug substances. Its effectiveness is attributed to its properties – PEG 200 is a low molecular weight surfactant that exists in a liquid state.4
The practical application of PEGs requires an understanding of their influence on the properties of administered drugs. One method for assessing this interaction is measuring the conductivity of the drug substance in a system containing the polymer. Previous studies, including those conducted by Italian researchers, have analyzed the molar conductivities of various PEGs, such as PEG 400 and PEG 2000, in the presence of sodium chloride (NaCl) solutions.5 Studies of this kind are particularly important due to the necessity of maintaining an isosmotic environment with the site of drug administration in certain dosage forms.6 The planned values of osmotic pressure can also be used in the development of new drug dosage forms intended for the controlled release of medicinal substances.7 In addition, conductometric techniques can subsequently be employed to study the release of substances from pharmaceutical systems containing polymeric drug carriers, representing a promising area of research.8, 9
Objectives
The aim of the study was to investigate the effect of selected polymers, i.e., PEG 200 and PEG 4000, on the physicochemical parameters of model systems containing 0.9% and 9.0% NaCl, and in particular to investigate the effect of these polymers on the specific conductivity of these systems. PEG 200 and PEG 4000 were selected due to their range of molar masses and melting temperatures, differing by about 100 K, and amounting to –50.0°C and 55.95°C, respectively, which ensured a different state of matter under the test conditions.10, 11 Moreover, we aimed to build upon the results previously obtained by Italian researchers,5 who studied the electrical conductivity of NaCl in the presence of polymers with similar properties, and to additionally investigate the effect of higher NaCl concentrations to more precisely assess the impact of these polymers on markedly hyperosmotic NaCl solutions in future studies.
Materials and methods
Materials
The following substances were used to prepare the tested solutions: PEG 200 and PEG 4000 (for synthesis; Merck Life Science, Poznań, Poland), distilled water from a deionization station (Hydrolab HLP20UV device; Hydrolab, Straszyn, Poland) and NaCl (pure p.a.).
Methods
Preparation of solutions
The composition of the tested solutions is presented in Table 1. Four series of solutions containing a constant amount of NaCl were prepared: 2 series with 0.9 g of NaCl per 100 g of solution, and 2 series with 9.0 g of NaCl per 100 g of solution, along with reference solutions containing only NaCl at concentrations of 0.9% and 9.0%, respectively, without any polymers. Each of the test series contained 7 mixtures with PEG 200 or PEG 4000. The amounts of PEG 200 or PEG 4000 used in both variants were: 2.0, 4.0, 6.0, 10.0, 15.0, 20.0, and 25.0 g.
The molar concentration of NaCl in the tested solutions (c) was determined based on the density measurements (ρr) of the systems, taking into account the total mass of the solution (mr) and the number of moles of NaCl (n), as shown in Equation 1:
Specific and molar conductivity of the tested solutions
The specific conductivity of the systems was measured using a CC-505 conductivity meter with an EC-70 conductivity sensor (k = 1.0 cm–1; Elmetron, Zabrze, Poland). The molar conductivity of the solutions (Λ) was calculated based on the measurements of specific conductivity (κ) and the molar concentration of NaCl (c), as shown in Equation 2.
Density and viscosity of the tested solutions
The other parameters, i.e., the density and viscosity of the tested systems, were measured using a Radwag AS X2 analytical balance (Radwag, Radom, Poland) with
a sinker of 9.9206 cm3, and an Ostwald viscometer. The
measurements were performed at a constant temperature of 25 ±0.5°C, using a thermostat.
Results
Specific and molar conductivity of the tested systems
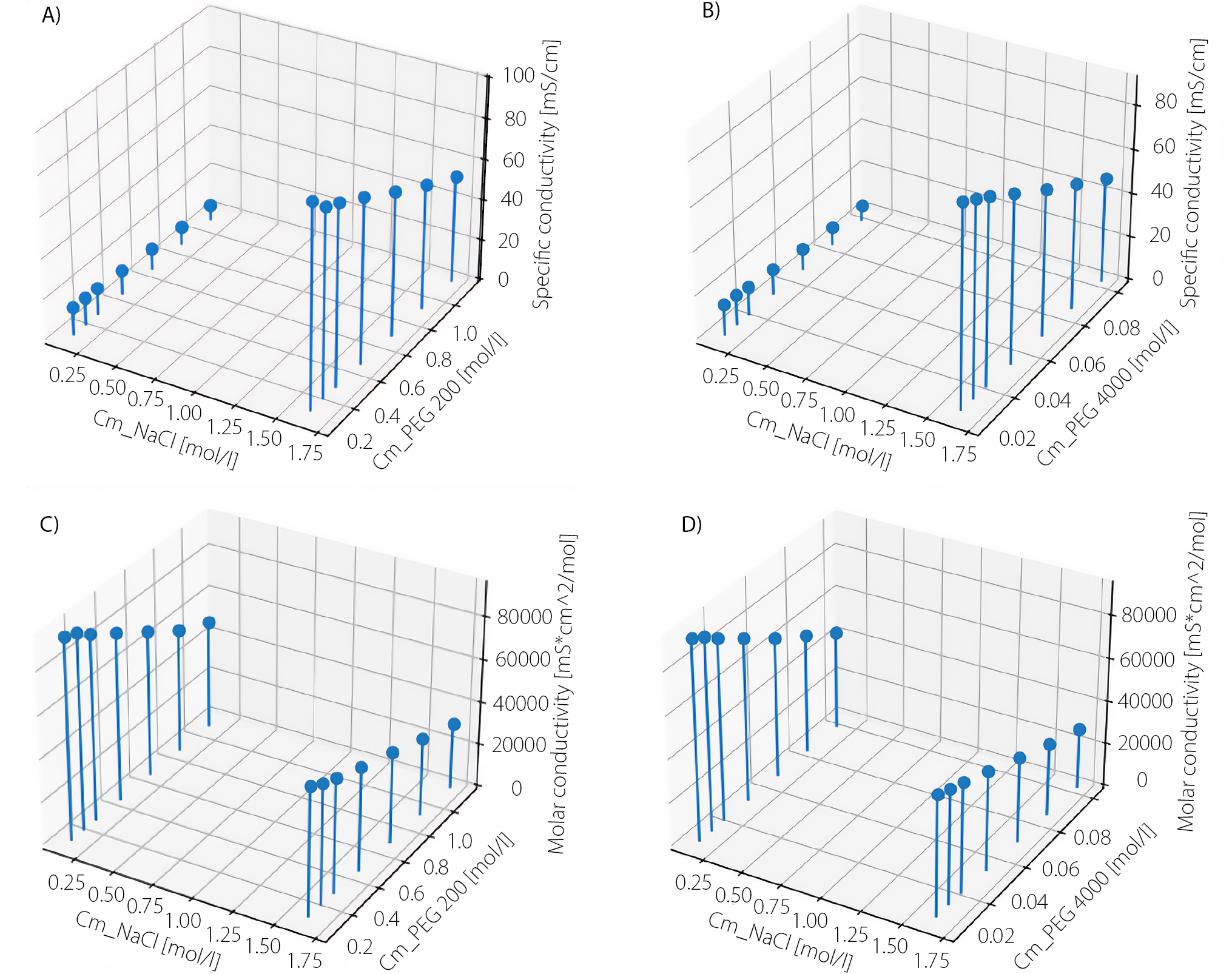
Figure 1 shows the changes in specific and molar conductivity with increasing concentrations of PEG 200 or PEG 4000 in 0.9% or 9.0% NaCl solutions.
A clear decrease in conductivity was observed with increasing polymer concentration in the NaCl solutions. In systems containing 0.9% NaCl, the conductivity of the tested solutions decreased from 14.60 to 8.28 mS/cm with increasing concentrations of PEG 200 (Figure 1; NaCl molar concentration below 0.25 mol/L). In the case of PEG 4000, conductivity decreased from 14.47 to 7.47 mS/cm (Figure 1; NaCl molar concentration below 0.25 mol/L). Significantly higher conductivity values were observed in the 9.0% NaCl solutions, ranging from 98.90 to 53.53 mS/cm (Figure 1) and from 91.30 to 48.73 mS/cm (Figure 1), corresponding to NaCl molar concentrations above 1.5 mol/L in both cases.
In 0.9% NaCl solution, the molar conductivity values decreased from 94,238.12 to 51,581.89 mS · cm2 · mol–1 with the addition of PEG 200 (Figure 1; NaCl molar concentration below 0.25 mol/L). For PEG 4000, the values ranged from 93,341.65 to 46,305.20 mS · cm2 · mol–1 under the same conditions (Figure 1, Figure NaCl molar concentration below 0.25 mol/L). In 9.0% NaCl solutions, the molar conductivity values ranged from 60,279.46 to 31,509.67 mS · cm2 · mol–1 (Figure 1) and from 55,665.02 to 28,562.21 mS · cm2 · mol–1 (Figure 1), respectively, at NaCl molar concentrations above 0.25 mol/L.
Viscosity of the tested systems
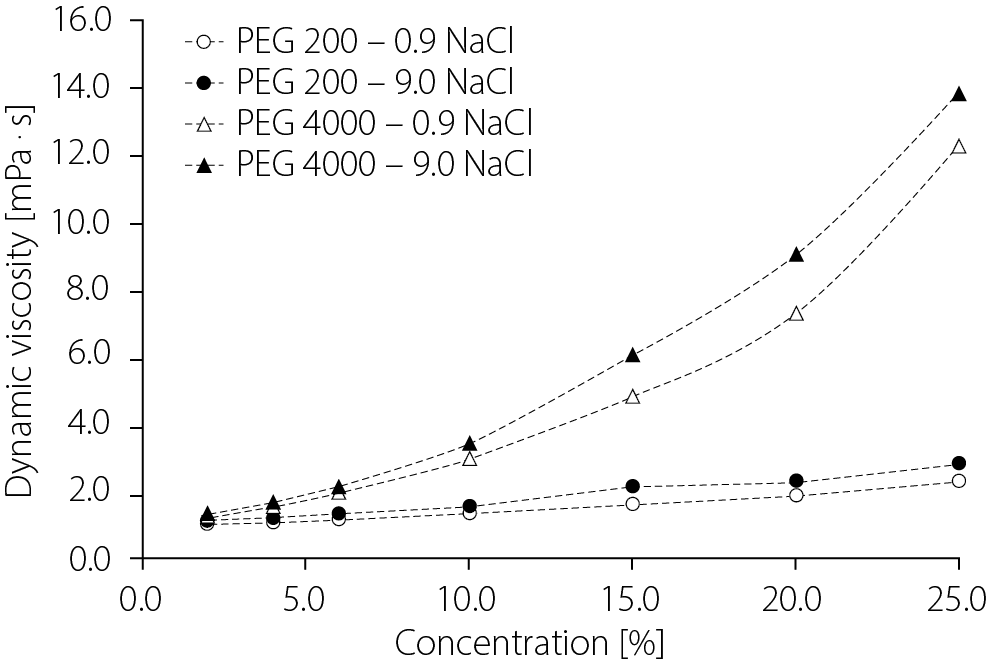
In contrast to conductivity, viscosity increased with increasing polymer concentration, as shown in Figure 2. Small differences were observed between systems containing 0.9% and 9.0% NaCl. The viscosity of the 0.9% NaCl formulations was in the range of 1.0104–2.2642 Pa · s (PEG 200) and 1.2054–12.2376 Pa · s (PEG 4000). In the 9.0% NaCl formulations, the values were 1.0941–2.8036 Pa · s (PEG 200) and 1.2958–13.8077 Pa · s (PEG 4000).
Discussion
The influence of concentration on the specific and molar conductivity of the tested systems
The study assessed the influence of the molar concentration of PEGs, PEG 200 and PEG 4000, on the specific and molar conductivity of NaCl solutions with weight concentrations of approx. 0.9% and 9.0%, as shown in Figure 1. The concentration of the first solution corresponded to that of physiological saline, with an osmotic pressure similar to that of body fluids. The 2nd physiological saline solution, with a tenfold higher NaCl concentration, was intended to confirm the effect of PEGs on the conductivity of the NaCl solution. It should be noted, however, that the increasing amounts of PEG 200 or PEG 4000 raised the density of the systems, resulting in a series of NaCl solutions with progressively higher molar concentrations of the salt. It was observed that, as a result of this increase in molar concentration, while maintaining the percentage concentration at 0.9%, the molar concentration rose by only 0.035–0.036 of the original value within the tested range. Therefore, this change was not significant for evaluating the effect of increasing polymer concentrations on the conductivity of NaCl solutions. A similar effect was observed in the case of the 9.0% NaCl solution, where the molar concentration increased by a fraction of 0.034 in both PEG 200 and PEG 4000 systems. The highest molar concentration of NaCl was recorded for the PEG4NaCl9-25 solution (1.699 mol/L), and the lowest for the PEG2NaCl09-2 solution (c = 0.155 mol/L). In the case of solutions without PEG addition, these concentrations were 0.154 mol/L (PEG0NaCl09) and 1.635 mol/L (PEG0NaCl9), respectively. A significantly greater influence of the polymers on conductivity measurements was observed in the higher-concentration NaCl solutions. According to the presented results, the viscosity of the system, resulting from increasing polymer concentrations, was a key factor influencing conductivity measurements. The data demonstrated that PEG concentration has a systematic effect on both specific and molar conductivity.
The relationship between molar conductivity and electrolyte concentration
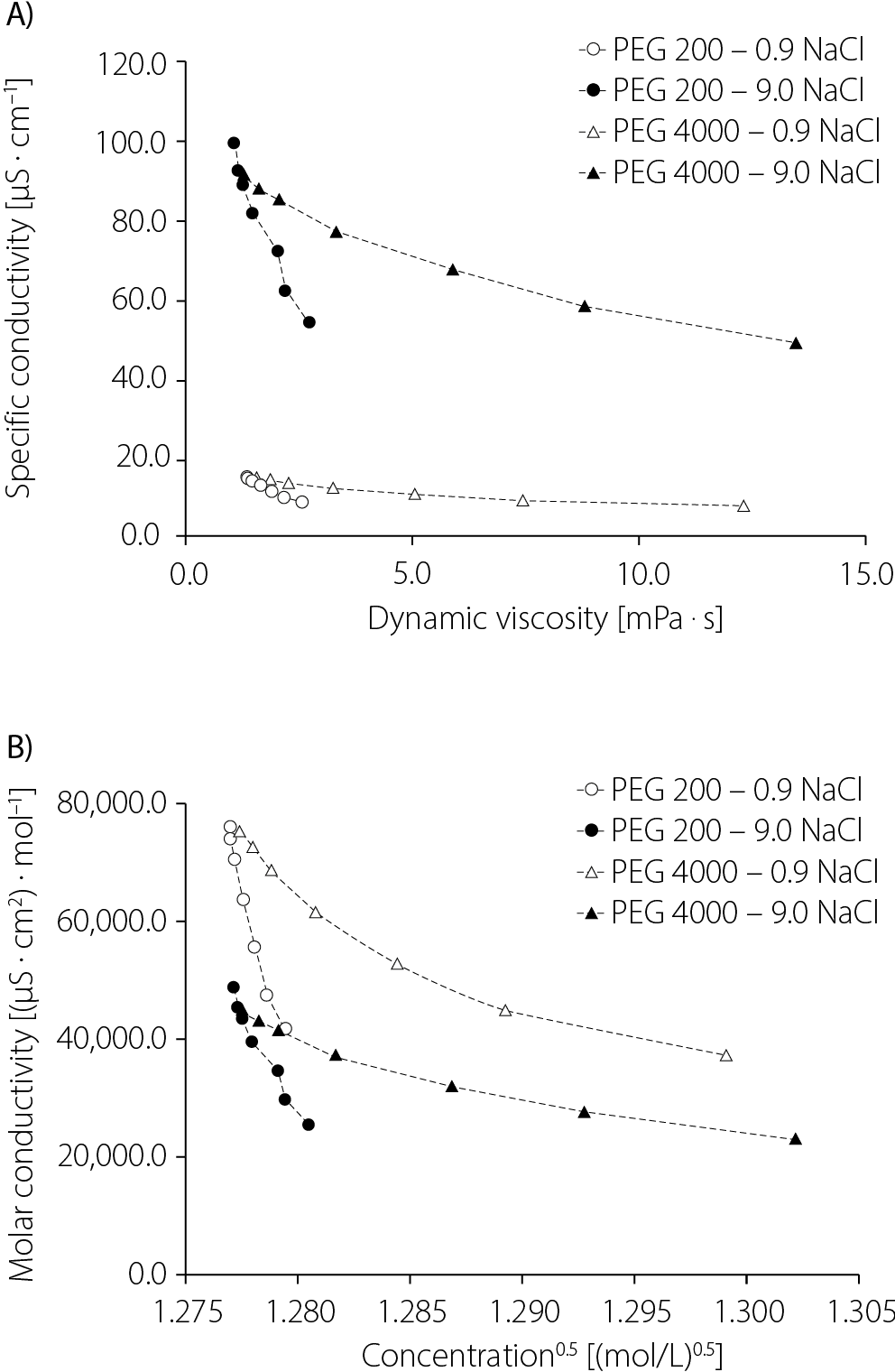
Figure 3 and 3B present the changes in molar conductivity, calculated from conductivity measurements, as a function of the elemental molar concentration of NaCl in the solutions.
The course of these graphs confirms that the studied systems do not deviate from the relationship proposed by Kohlrausch.12 In this relationship, the molar conductivity of the electrolyte (Λ) is a function of concentration (c), and the limiting molar conductivity (Λ₀) is represented graphically by the y-intercept of the extrapolated curve (Equation 3). The coefficient a is characteristic of the studied system and was originally interpreted by Debye and Hückel13 and later reinterpreted by Onsager14:
Extrapolating the graphs to the x-axis can provide information on the limiting molar conductivity values in the studied systems. Indeed, the cut-off points of the extrapolated courses of the abovementioned relationship are similar to each other in solutions with the same NaCl concentrations. Significantly higher values of the cut-off points were noted for lower NaCl concentrations, and the polymer with a higher molar mass favored a slight decrease in electrolytic conductivity. It should be noted that the tested range of NaCl concentrations was small and requires possible confirmation in tests conducted on a wider range of NaCl concentrations.
The influence of viscosity on the specific and molar conductivity of the tested systems
An interesting relationship was observed when comparing viscosity with specific and molar conductivity (Figure 4)
Both specific and molar conductivity decreased with increasing viscosity. However, a sharper decrease was observed with PEG 200. The decrease in conductivity with increasing viscosity was less pronounced in the case of PEG 4000. This phenomenon may be attributed to differences in both the concentration and molar mass of the polymers used.
The importance of conductivity and viscosity for the practical application of the tested mixtures
The use of polyoxyethylene glycols as carriers and substrates for medicinal substances is particularly important in the context of electrical conductivity studies. Due to the need to maintain appropriate osmotic pressure in certain cases, corresponding to a 0.9% NaCl solution, conductivity can serve as an informative parameter, providing insight into the properties of such systems. In addition, PEG-water mixtures are characterized by a high potential for increasing the solubility of some chemical compounds. For example, to enhance the solubility of the non-steroidal anti-inflammatory drug etoricoxib, PEG 400 was used as a cosolvent for oral administration.15 PEG 4000 has been similarly applied to enhance the solubility of carbamazepine,16 while PEG 6000 has been used for dihydroartemisinin.17 The increase in solubility is also important in systems characterized by a specific osmotic pressure. Hence, it was interesting to determine the value of electrical conductivity in the context of adding cosolvent polymers to an isosmotic solution.
The observed results indicate that, regardless of the type of PEG used or the NaCl concentration, molar conductivity decreases linearly with increasing electrolyte concentration. This confirms the principle of a linear relationship between the molar conductivity of a strong monovalent electrolyte and the square root of its molar concentration.18 Based on a 2007 study by Capuano et al. which examined polyoxyethylene glycols of similar molecular weight (PEG 4, PEG 400, PEG 2000), it is known that the addition of polyethylene glycol to aqueous NaCl solutions does not influence ion–ion electrostatic interactions. Taking this knowledge into account, in subsequent stages of research, it is possible to investigate, among others, the values of the limiting molar conductivity of NaCl solutions in PEG-water mixtures in a wider range of concentrations, and the effect of medicinal substances on the conductivity of such a system.5
Conclusions
The addition of a polymer to a system containing NaCl at concentrations of 0.9% or 9.0% increases the solution viscosity. The increase in viscosity increases the calculated molar concentration of NaCl. With the increase in the calculated molar concentration of NaCl, the molar conductivity decreases proportionally according to the Kohlrausch equation. The rate of change in molar conductivity with the change in concentration is greater in the case of a system containing 9.0% NaCl. PEG 200 and polyoxyethylene glycol 4000 similarly influence the pattern of conductivity changes in both 0.9% and 9.0% NaCl solutions. The aim of future studies will be to determine the effect of the tested polymers on the estimated values of limiting molar conductivity.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.