Abstract
Background. Poly(glycerol sebacate) is a polymeric material with potential biomedical application in the field of tissue engineering. In order to act as a biodegradable scaffold, its incubation study is vital to simulate its behavior.
Objectives. This study explores the degradation of porous poly(glycerol sebacate)/hydroxyapatite scaffolds subjected to incubation in various physiological solutions.
Materials and methods. The research involved monitoring pH and conductivity values over a 14-day period, as well as analyzing the swelling capacity and mass alterations of the scaffolds.
Results. In simulated body fluid (SBF) and phosphate-buffered saline (PBS), the pH levels remained relatively stable, whereas Ringer’s solution caused a pH decrease. Conversely, artificial saliva demonstrated an increase in pH, and distilled water caused a slight decrease. The conductivity values remained stable in SBF and Ringer’s solution, slightly decreased in PBS, increased in artificial saliva, and significantly increased in distilled water. The swelling capacity of the scaffolds varied depending on the solution used, with the lowest equilibrium swelling observed in SBF and PBS. The effect of the presence of ceramics on this parameter was also observed. The mass changes of the scaffolds indicated deposition of particles or salts from the incubation solutions, and subsequent rinsing in distilled water led to a decrease in mass. Scanning electron microscopy (SEM) imaging and elemental analysis confirmed the presence of crystallized salts on the scaffold surfaces after incubation in SBF. Surface roughness measurements revealed changes in roughness depending on the solution, with deposition of additional layers in SBF and degradation in artificial saliva.
Conclusions. In summary, the scaffolds exhibited biodegradation in physiological solutions, with variations in pH, conductivity, swelling capacity, mass changes, and surface morphology depending on the specific solution and scaffold composition.
Key words: hydroxyapatite, incubation, poly(glycerol sebacate), scaffolds
Streszczenie
Wprowadzenie. Poli(sebacynian gliceryny) to materiał polimerowy o potencjalnym zastosowaniu biomedycznym w dziedzinie inżynierii tkankowej. Aby efektywnie pełnił rolę biodegradowalnego rusztowania komórkowego, niezbędne jest przeprowadzenie badania inkubacyjnego w celu symulacji jego zachowania.
Cel pracy. Prezentowane badanie przedstawia zachowanie pianek z poli(sebacynianu gliceryny) oraz hydroksyapatytu podczas inkubacji w różnych roztworach fizjologicznych.
Materiał i metody. Badania obejmowały monitorowanie wartości pH i przewodności przez okres 14 dni, a także analizę pęcznienia i zmian masy materiałów.
Wyniki. W buforze SBF (ang. simulated body fluid) oraz PBS (ang. phosphate-buffered saline) wartość pH pozostawała na stałym poziomie, podczas gdy w płynie Ringera wartość pH ulegała obniżeniu. Podczas inkubacji w sztucznej ślinie wartości pH wrastały, a w wodzie destylowanej ulegały niewielkiemu obniżeniu. Wartości przewodności pozostawały stabilne w SBF i płynie Ringera. Uległy natomiast niewielkiemu spadkowi w PBS, wzrosły w sztucznej ślinie, a znacznie wzrosły w wodzie destylowanej. Pęcznienie pianek ma bazie PGS zależało od użytego roztworu. Najniższe wartości pęcznienia równowagowego zaobserwowano w SBF i PBS. Zmiany masy rusztowań wskazywały na osadzanie się cząstek lub soli z roztworów inkubacyjnych. Przepłukanie materiałów wodą destylowaną powodowało obniżenie masy. Obrazowanie techniką skaningowej mikroskopii elektronowej (SEM) oraz analiza elementarna potwierdziły obecność soli wykrystalizowanych na powierzchniach materiałów po inkubacji w SBF. Pomiar chropowatości wykazał zmiany w wartościach współczynnika Sa w zależności od rodzaju wykorzystanego roztworu. W SBF dochodziło do depozycji dodatkowych warstw apatytowych oraz do zwiększonej degradacji w sztucznej ślinie.
Wnioski. Rusztowania wykazywały biodegradację w roztworach fizjologicznych. Różnice w przebiegu inkubacji były ilustrowane pomiarami wartości pH, przewodności, zdolności do pęcznienia, zmianie mas oraz morfologii powierzchni. Różnice wynikały z wykorzystanego roztworu oraz składu pianki.
Słowa kluczowe: hydroksyapatyt, inkubacja, poli(sebacynian glieryny), scaffoldy
Introduction
Poly(glycerol sebacate) (PGS) is an emerging biodegradable polyester for biomedical application, foremost in tissue engineering.1, 2, 3, 4 This polymeric material is considered biodegradable,5, 6 which is a desired trait for modern biomedical application. However, a biomaterial can behave diversely in various physiological fluids. In this study, PGS was combined with bioactive hydroxyapatite (HAp) in order to obtain porous scaffolds for bone tissue regeneration. This ceramics was selected considering its impressive properties in terms of its impact on osseointegration processes. Hydroxyapatite is widely applied in dentistry as well as orthopedics for its similarity in chemical composition to the inorganic phase of bone.7, 8, 9 For this reason, as well as because of its osteoconductive properties, HAp is used in tissue engineering, with both in vitro and in vivo applications widely reported in the literature.10 A material exhibiting osteoconductivity ensures the appropriate environment for the ingrowth of bone-forming elements from the surrounding area. Furthermore, by stimulating osteoblasts to proliferate, the growth of new apatite layers occurs.11, 12 Therefore, PGS is a great addition to biomaterials for bone regeneration, especially polymers, which often display significantly lower mechanical strength.13, 14
In order to simulate the behavior of biomaterials in living organisms, different types of artificial biological fluids are used, of which the most common are simulated body fluid (SBF), artificial saliva, Ringer’s fluid (which corresponds to the composition of extracellular fluid), or phosphate-buffered saline (PBS), whose composition corresponds to body fluids.15, 16, 17 Different pH values as well as the composition of various artificial biological fluids affect the behavior of biomaterials in their presence, and in the case of in vitro tests, they can quickly provide the first information.18, 19 Such incubation studies are particularly important in the context of materials that are assumed to degrade in the body environment. Detailed potentiometric as well as conductometric monitoring helps to determine whether, during the process of degradation of the material into finer elements, there is precipitation and/or release of other components that could negatively affect the cellular balance. Strong acidification, i.e., a spike in pH values to highly alkaline, is a cause for concern, as under such conditions, cells in the area of the implant are unable to proliferate.20, 21 Therefore, in vitro studies are advisable before proceeding to cellular or in vivo studies on animal models.
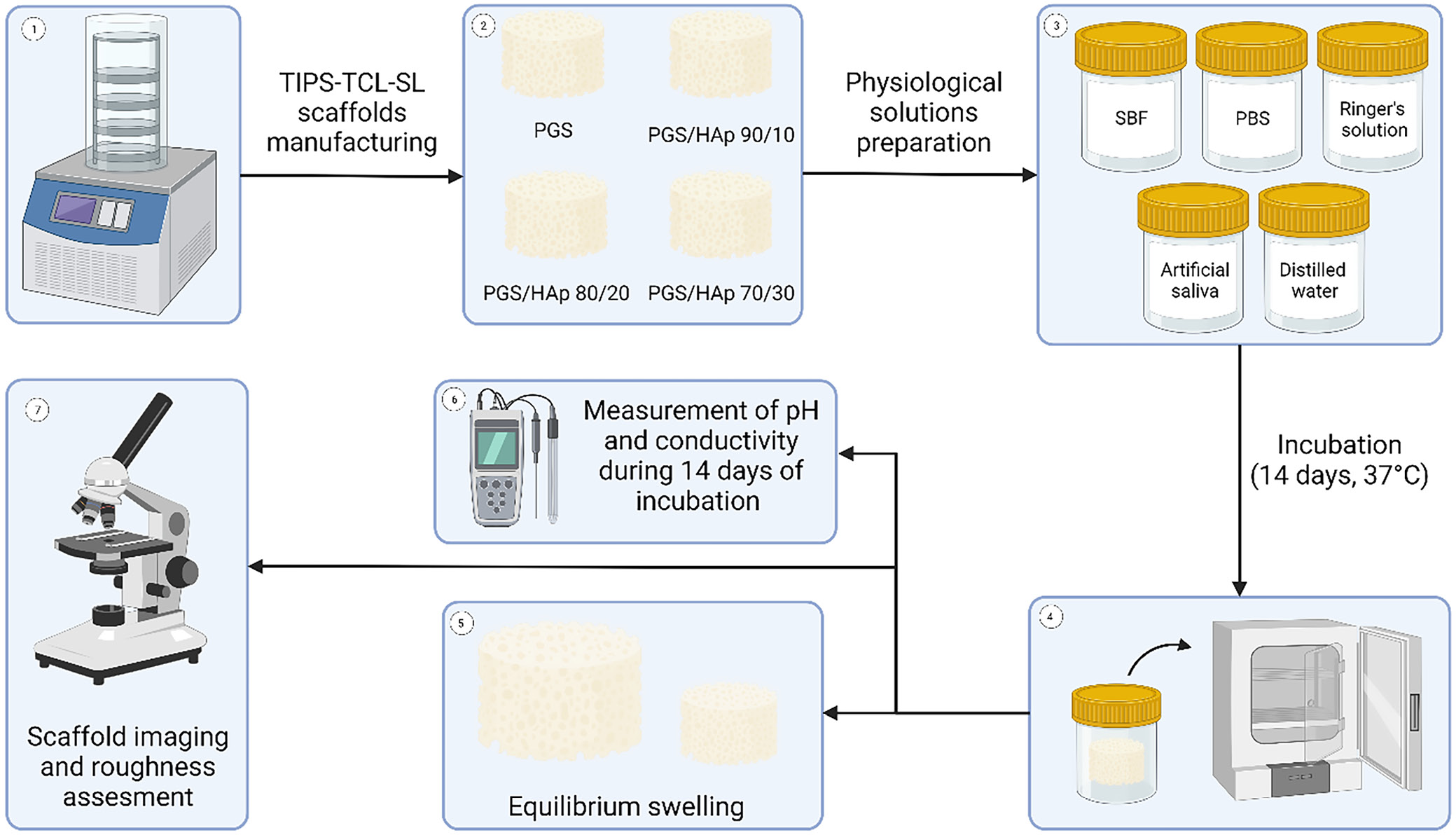
As the physico-chemical and mechanical properties as well as cytocompatibility with L929 fibroblast cells of the very same materials was reported previously,22 this study puts an emphasis on the behavior of the PGS and PGS/HAp scaffold materials during incubation. It is crucial to investigate the behavior of the biomaterial in various conditions and incubation fluids in order to consider further clinical application. Graphical scheme of the study is presented in Figure 1.
Materials and methods
All of the chemical compounds utilized for synthesis of HAp and obtaining buffers were obtained from Chempur (Piekary Śląskie, Poland), except ammonia solution, which was obtained from Stanlab (Lublin, Poland).
Synthesis of HAp
The synthesis process followed a previously reported wet precipitation method protocol.23, 24 First, 3.63 g of Na2HPO4 was dissolved in 80 mL of distilled water. The solution was transferred to the 3-necked, round-bottomed flask equipped with condenser and a thermocouple. Subsequently an additional 520 mL of water was added to dilute the solution. To achieve a pH of 11, a few drops of 25% ammonia solution were carefully added. After boiling the water, a solution of Ca(CH3COO)2 (created by dissolving 4.51 g of salt in 200 mL of water) was added dropwise with rate of 1 drop/s. After the solution was added, the reaction mixture was cooled to the ambient temperature and transferred to the beaker for sedimentation in the course of 24 h. The precipitate was subsequently washed with water until it reached a neutral pH. After centrifuging, the supernatant was decanted and obtained HAp was submitted to freeze-drying (−50°C, p < 10 Pa) after subsequent freezing (−15°C).
pPGS prepolymer synthesis and TIPS-TCL-SL scaffold manufacturing
The synthesis of poly(glycerol sebacate) prepolymer (pPGS) followed a previously established procedure by the authors.22, 25 Briefly, sebacic acid and glycerol were combined in an equimolar ratio with glycerol in 130°C. Reaction was allowed to proceed for 24 h and was halted by reducing the temperature to 25°C. Porous PGS scaffolds containing 0, 10, 20, and 30 wt% of HAp were manufactured using thermally induced phase separation followed by thermal cross-linking and salt leaching (TIPS-TCL-SL) technique described previously.22, 23 In brief, pPGS was dissolved in 1,4-dioxane at a concentration of 20 wt% and the corresponding amount of HAp was added to the mixture (with respect to the prepolymer mass) and stirred for 24 h. Solution was poured onto the porogen (400–500 µm NaCl particles) in a multi-well plates and frozen overnight. Afterwards, the specimens were freeze-dried and cured at 130°C for 7 days. After cross-linking, the porogen was leached out with water and samples were dried before subjecting for further experiments.
The authors had previously conducted physicochemical and structural characterization of the prepolymer22, 23, 25 which included identification of characteristic structural bands on Fourier-transform infrared spectroscopy (FT-IR) spectrum, structural characterization with nuclear magnetic resonance (MRI) technique and contact angle measurement. Furthermore, the porous scaffolds manufactured in the same conditions were also characterized with respect to their thermal and mechanical properties.22
Buffer preparation and scaffold incubation
The scaffolds underwent a 14-day incubation period in 5 different physiological solutions: SBF, PBS, Ringer’s solution, artificial saliva, and distilled water. A detailed breakdown of the composition of the utilized buffers can be found in Table 1. Solutions were obtained by dissolving the appropriate ingredients in distilled water except for the PBS solution, which was prepared by dissolving in water a ready-made commercial product in the form of tablet (Oxoid; Thermo Fisher Scientific, Waltham, USA). The SBF solution was prepared in the sequence presented in the Table 1 in 36.5°C. After all ingredients were dissolved, 1M HCl was added until pH was in the range of 7.5–8.0. The rest of the solutions were prepared in the room temperature. Four scaffolds of each type were placed in separate sterile 100 mL containers and poured over with 75 mL of each buffer. The incubation was performed in ST 5 SMART incubator (POL-EKO, Wodzisław Śląski, Poland) in 37°C for 14 days.
Buffer preparation and scaffold incubation
Throughout the incubation period, the solutions containing the scaffolds were regularly analyzed for pH and conductivity to investigate interactions between the samples and the incubation fluids. Conductivity and pH were assessed using a CX-701 pH-meter (Elmetron, Zabrze, Poland). These assessments were conducted at specific time intervals, namely, on the 1st, 3rd, 7th, 9th, and 14th day of the incubation process.
Equilibrium swelling
The swelling capacity of the tested scaffolds was assessed in all 5 incubation fluids over a 14-day period, with measurements taken at specific time intervals: 15 min, 30 min, 1 h, 2 h, 4 h, 1 day, 2 days, 7 days, 9 days, and 14 days. Samples with an initial mass ranging from 0.14 g to 0.22 g were placed in sterile 100 mL containers and submerged with 75 mL of the corresponding buffer solution. The swelling ratio (Sw) was calculated using Equation 1,24, 26, 27 where Wt is the mass of swollen sample after given time and W0 is the initial mass of the scaffold.
(1)
The swelling kinetics of the scaffolds was investigated using Voigt-based viscoelastic model using Equation 2,28, 29 where St is swelling ratio at given time (t), Se is equilibrium swelling and τ is a swelling rate parameter (time required to reach 0.63 of maximum swelling value).30, 31
(2)
The exponential fitting was performed in Origin software v. 2021b (OriginLab Corporation, Northampton, USA). The R2 Pearson’s correlation parameter was higher or equal to 0.92 within each fitting.
Optical imaging, 3D reconstruction and surface roughness measurement
Surface of the scaffolds were imaged using VHX Series Digital Microscope (Keyence, Osaka, Japan). Images were captured in 4k mode, providing a resolution of 4,000 × 3,000 pixels with additional depth of focus analysis. The CMOS VHZ-700 sensor (Keyence) enabled roughness analysis and the creation of 3D reconstructions of the scaffold surfaces. Scanning electron microscopy (SEM) microphotographs and local elemental analysis measured with energy dispersive spectroscopy (EDS) were registered using SEM Jeol 5510LV system (Jeol, Tokyo, Japan). Measurement parameters included threshold angle of 30° and voltage of 10 kV. The SEM microphotographs were registered with ×500 magnification. Before imaging the samples were spray-coated with gold using Cressington 108 sputter coater (Cressington Scientific Instruments, Watford, UK).
Results
Structural, physico-chemical and biological properties of porous PGS-based materials was reported by the authors previously.22, 23 Therefore, this article explores divergent area which is stability, swelling, mass loss, and imaging during in vitro incubation in 5 different physiological solutions. Furthermore, the parameters of HAp synthesized and utilized in the presented study, including surface specific area, crystallinity and imaging, were published previously.22, 32
Evolution of pH and conductivity during incubation
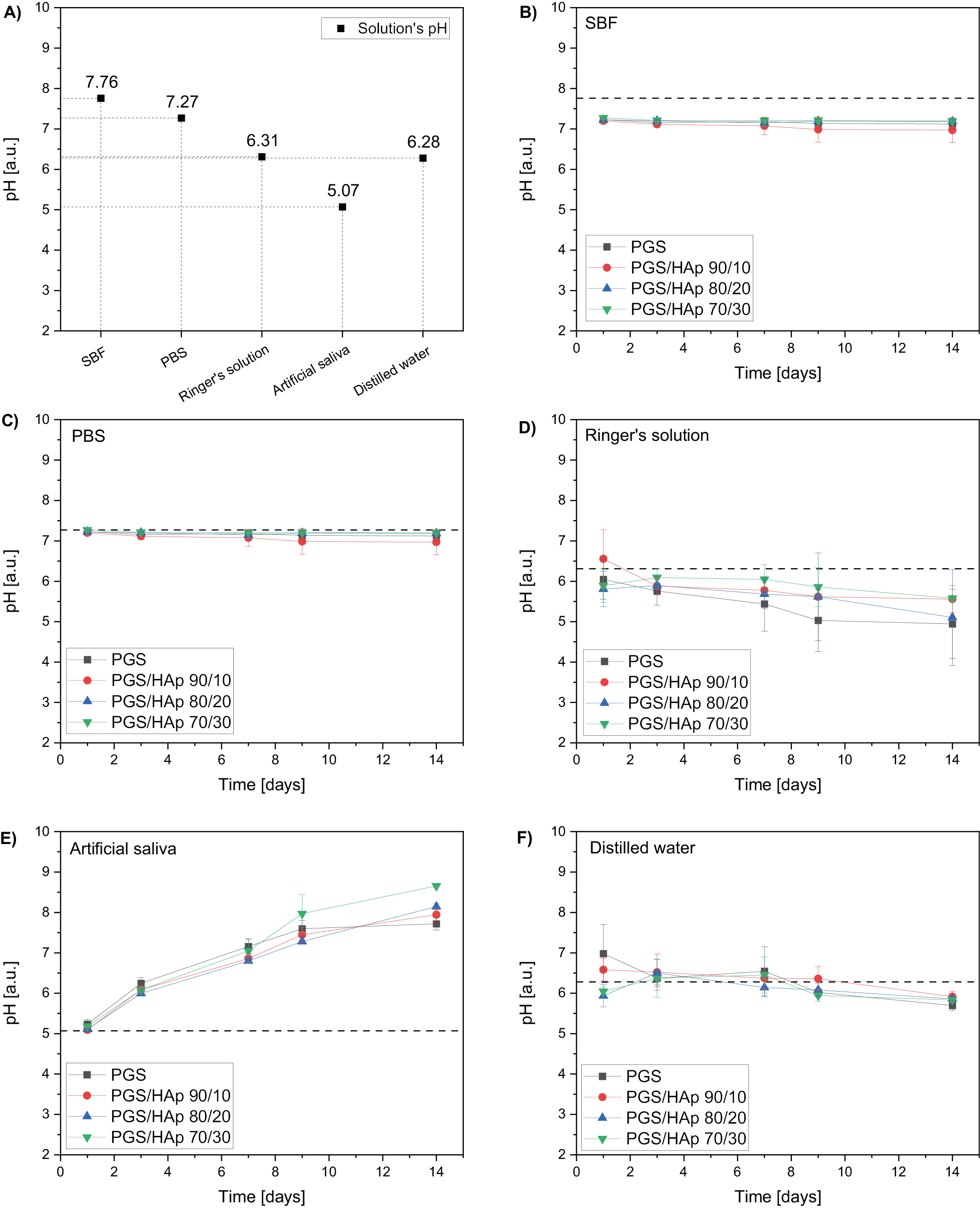
During an incubation of scaffolds in the physiological liquids the pH (Figure 2, Figure 3), conductivity values were registered after 1, 3, 7, 9, and 14 days. The reference pH values of the solutions were 7.76 for SBF, 7.27 for PBS, 6.31 for Ringer’s solution, 5.07 for artificial saliva, and 6.28 for distilled water. During the process, the pH values remained approximately constant for SBF and PBS. In the Ringer’s solution, the pH values lowered after 14 days to 4.90 for PGS, 5.56 for PGS/HAp 90/10, 5.10 for PGS/HAp 80/20, and 5.58 for PGS/HAp 70/30. The decrease was the biggest for PGS and PGS/HAp 80/20 samples. The reverse effect was observed for artificial saliva. The pH values of the solutions increased over the incubation time up to 7.70 for PGS, 7.90 for PGS/HAp 90/10, 8.10 for PGS/HAp 80/20, and 8.70 for PGS/HAp 70/30.
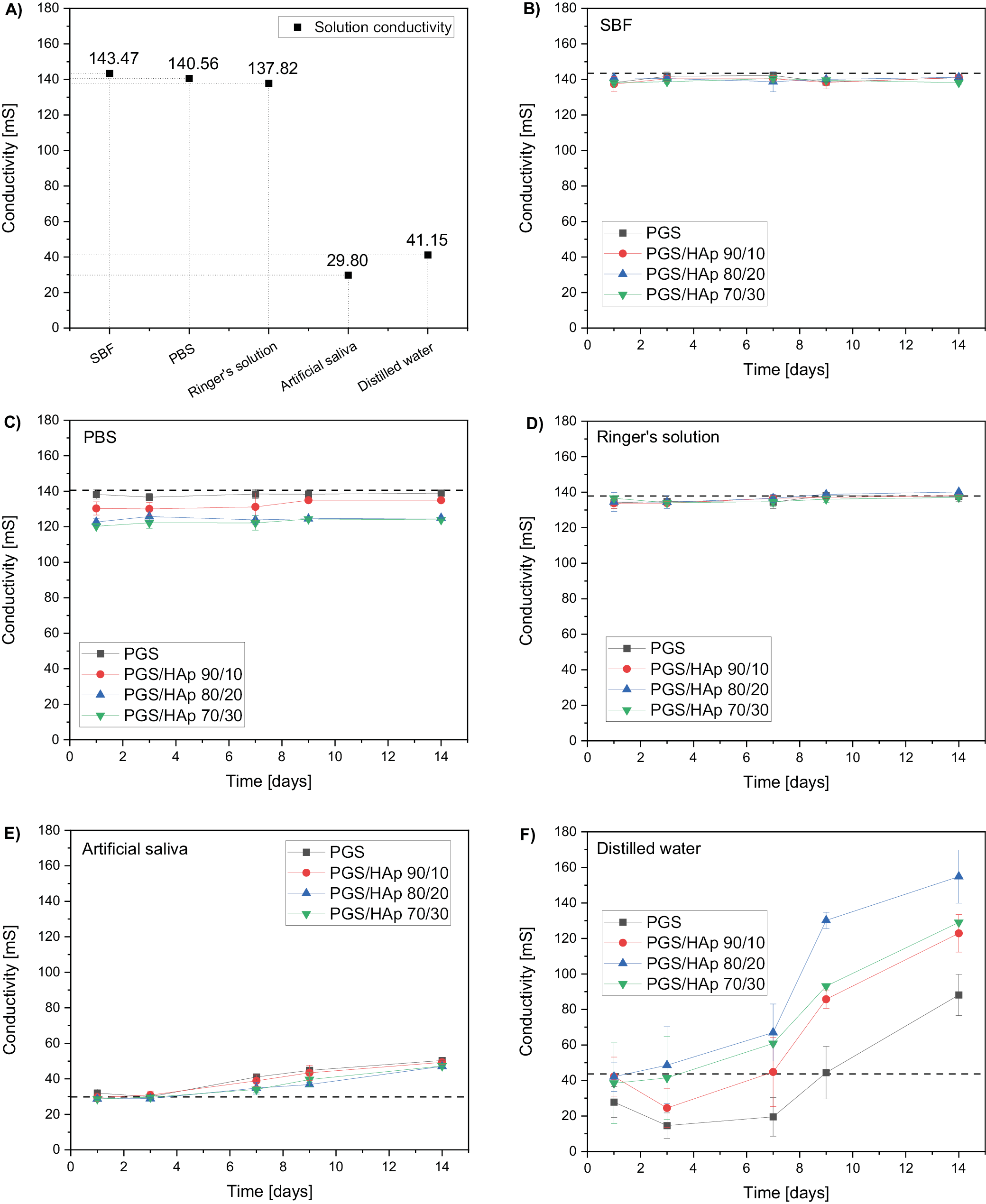
Values of conductivity change with the concentration of the ions during incubation in the environment of solutions imitating physiological conditions.33 Measured values for the reference solutions were 143.5 mS for SBF, 140.6 mS for PBS, 137.8 mS for Ringer’s solution, 29,8 mS for artificial saliva, and 41.2 mS for distilled water. The conductivity remained approximately constant for SBF and Ringer’s solution. In PBS, the measurements were also constant. However, they were slightly lower the buffer’s reference value. In artificial saliva, a slight increase in measured property was noted (from ~30 mS to ~50 mS for all materials). The highest increase was observed in distilled water (even by 400% for PGS/HAp 80/20).
Equilibrium swelling
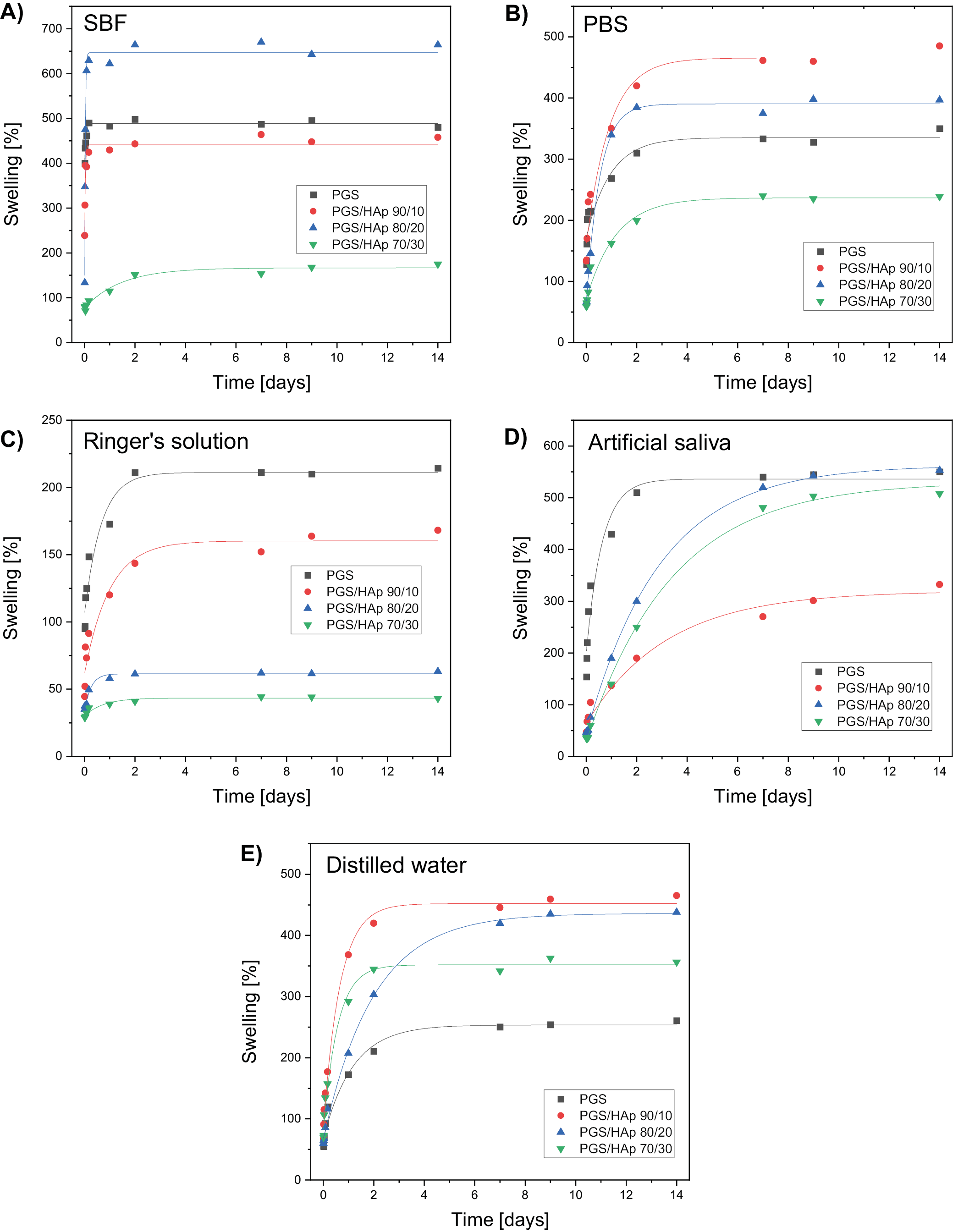
The swelling curves for all evaluated scaffolds were fitted using Equation 2 and presented on Figure 4. The corresponding values of Se and τ were juxtaposed in Table 2, Table 3. Swelling analysis was performed for all solutions utilized previously. Based on the evolution of pH and conductivity over time, physiological solutions can be divided into groups: 1) those in which neither pH nor conductivity changed statistically significant over time (G1: SBF and PBS); 2) those in which pH level dropped down during incubation while conductivity was not affected (G2: Ringer’s solution); and 3) solution in which both conductivity and pH values changed over the course of 14 days (G3: artificial saliva and distilled water). This division is beneficial for analyzing the swelling capacity of the scaffolds.
In G1, the material with lowest equilibrium swelling was PGS/HAp 70/30 with Se of 166.7% in SBF and 236.7% in PBS. It is worth mentioning that in SBF, except for PGS/HAp 70/30 sample, all other materials possessed the highest τ for all measured samples and therefore their swelling was the swiftest. In Ringer’s solution (G3) the Se swelling was the lowest among all solutions and gradually lowered with the concentration of HAp (from 211% for PGS down to 43% for PGS/HAp 70/30). The highest τ values (except for PGS) were observed in artificial saliva, indicating the highest time required to achieve full swelling capacity (3.20 for PGS/HAp 90/10, 2.91 for PGS/HAp 80/20 and 3.39 for PGS/HAp 70/30). In H2O, PGS exhibited lowest Se (211%) and PGS/HAp 90/10 the highest (452%). In G3 no direct correlations between pH, conductivity and Se is present.
Scaffolds’ imaging and roughness assessment
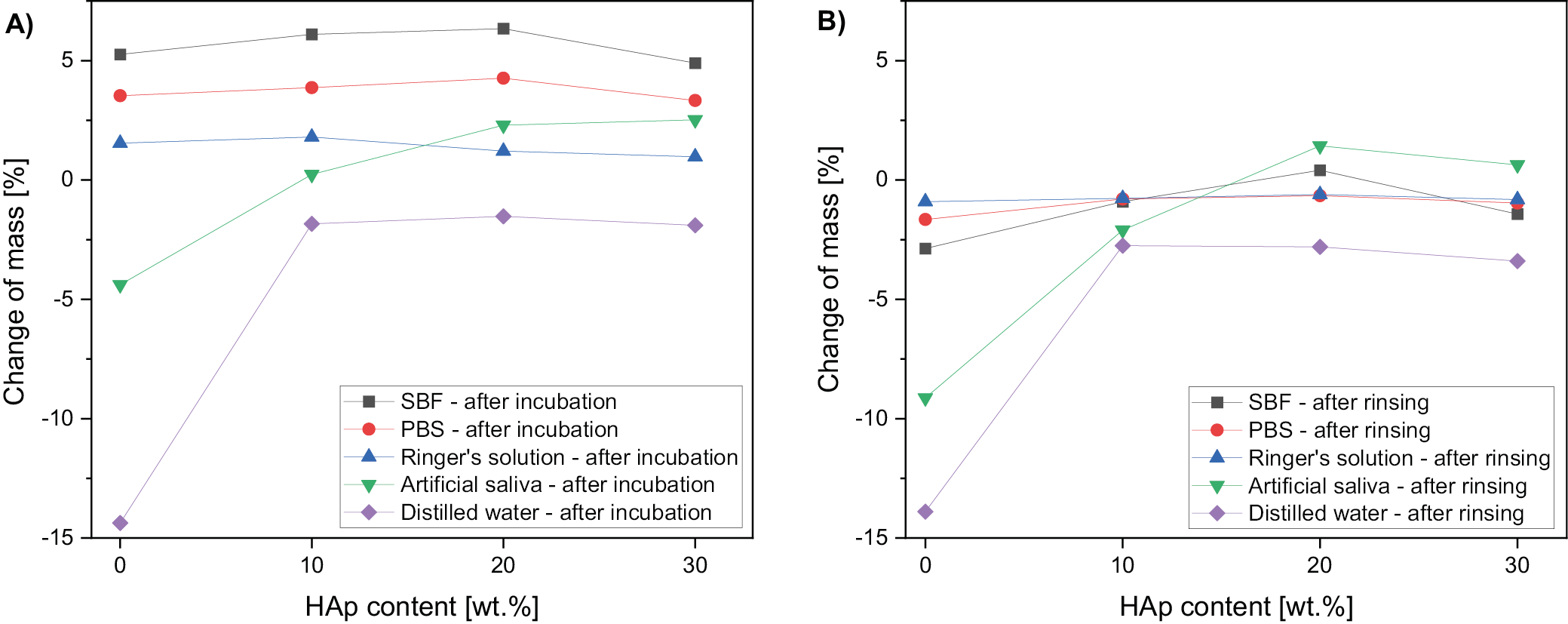
The change of scaffolds mass after the incubation is presented in Figure 5. Samples were weighted directly after drying from incubation solutions (Figure 5A) and after subsequent rinsing overnight in distilled water (Figure 5B). For all scaffolds incubated in SBF, PBS and Ringer’s solution, the change of mass was positive, weighted after drying straight from the incubation solutions.
After subsequent rinsing of the samples in water, the mass decreased in comparison to samples dried directly after incubation in all specimens and solution (Figure 5B). The change of mass was the most significant in distilled water and dropped to −15% for polymer scaffold.
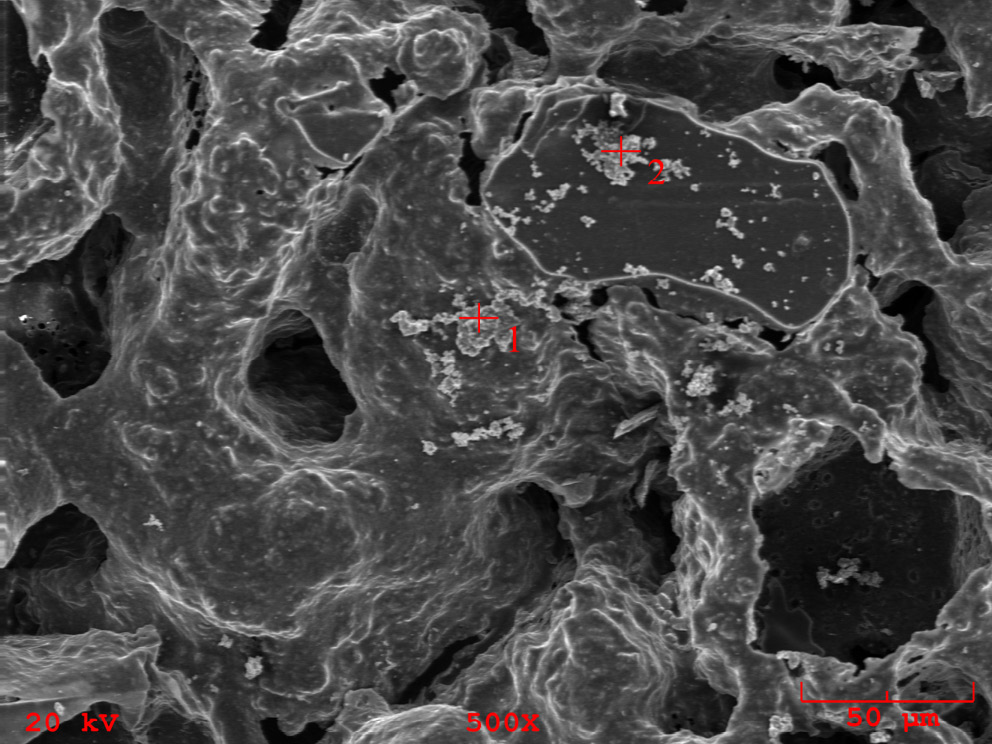
Moreover, the SEM imaging and EDS measurement revealed that for sample incubated in SBF, crystallized domains of salts present in the buffer composition were observed (Figure 6). The accurate areas of EDS measurements are indicated as red marks on the SEM image. The detailed elemental composition is presented in Table 4. Most importantly, the majority of abundance consists of Na and Cl elements not present in the initial sample before incubation. The EDS measurement and SEM imaging performed on reference PGS and PGS/HAp scaffolds before incubation showcased the morphology and confirmed presence of apatite filler.22
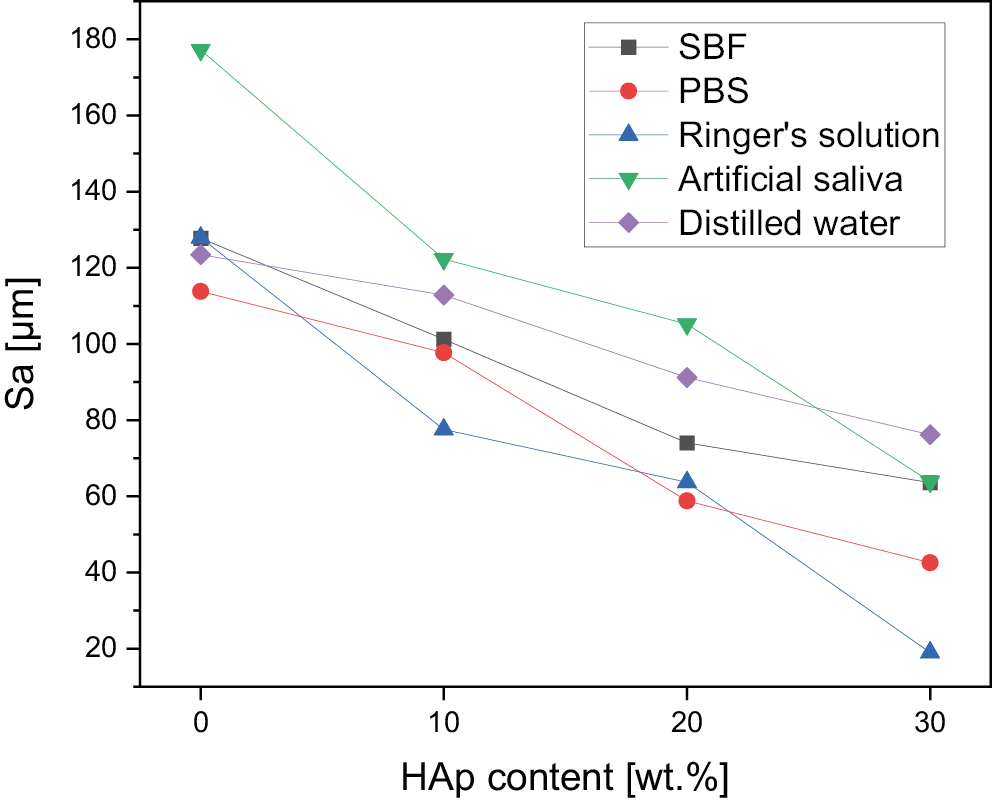
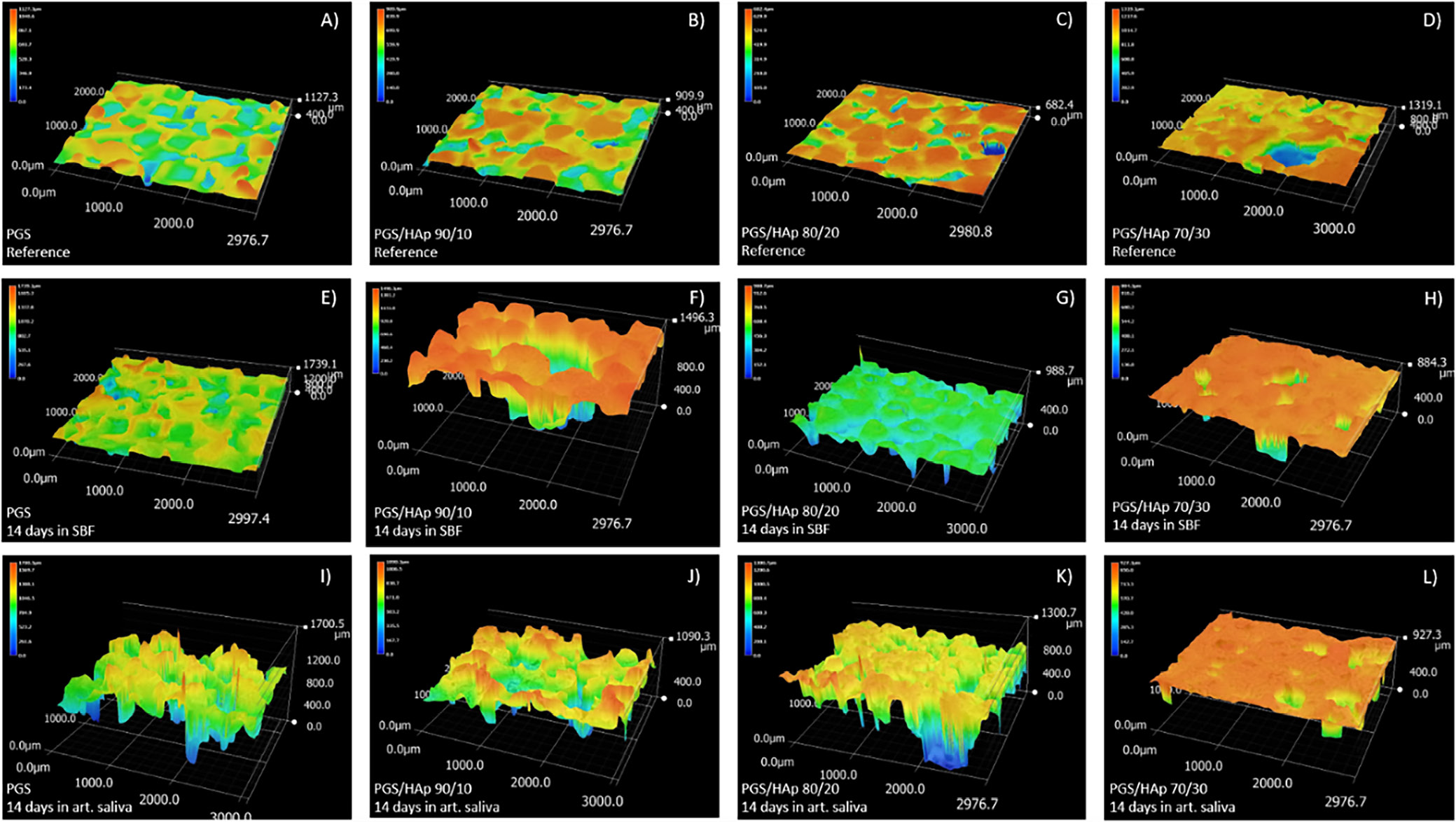
The morphological changes are depicted by the evolution of the surface roughness measurement basing on 3D reconstructions from the optical microscope (VHX Series Digital Microscope; Keyence) (Figure 7). For the reference samples before the incubation, a decrease in roughness can be observed as the amount of apatite filler increases: 132.2 μm for PGS, 116.6 μm for PGS/HAp 90/10, 89.0 μm for PGS/HAp 80/20, and 80.6 μm for PGS/HAp 90/30. This downward trend with the increasing amount of HAp persisted for all utilized solutions. Surface roughness before and after incubation is also presented in the Table 5.
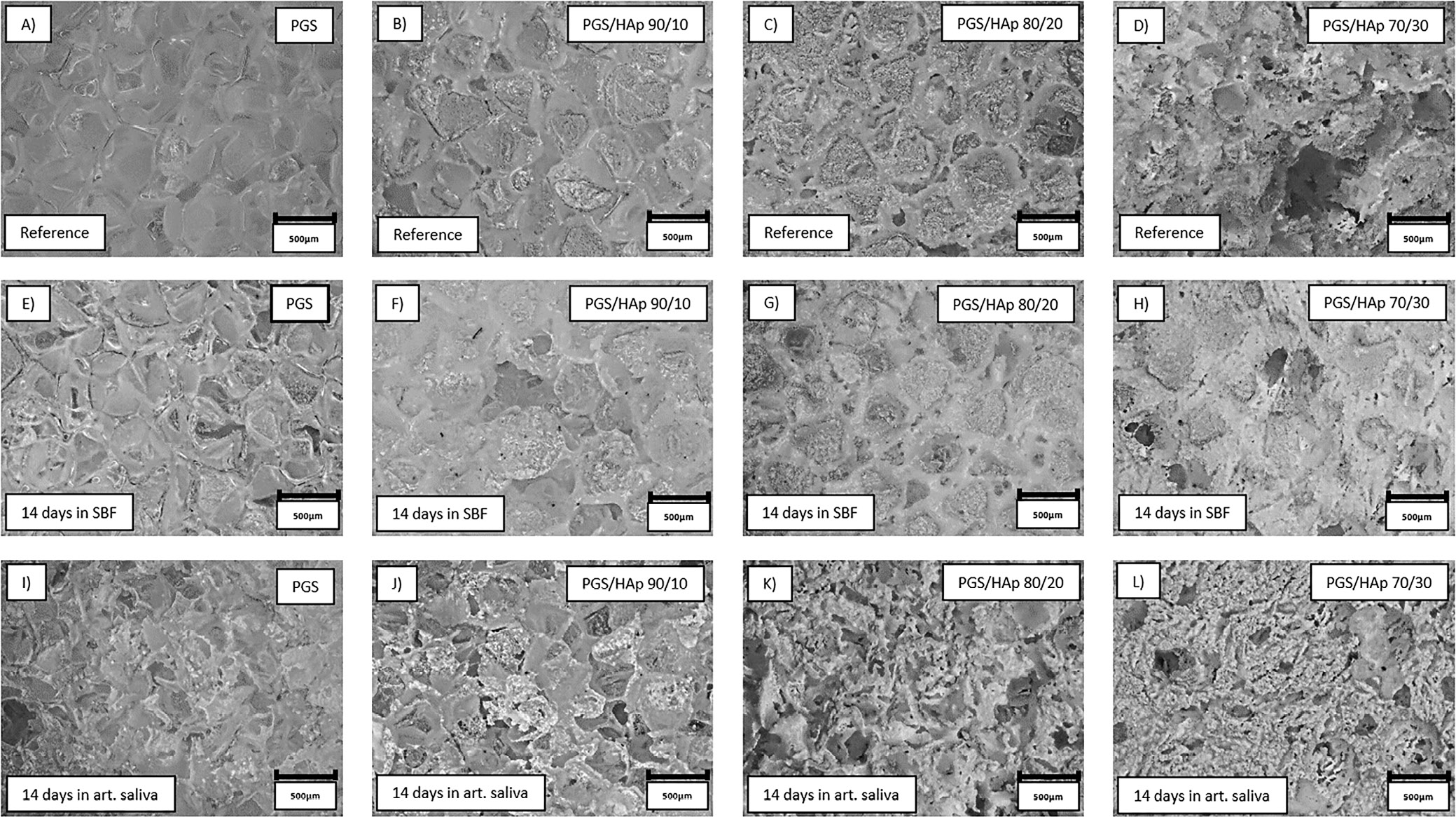
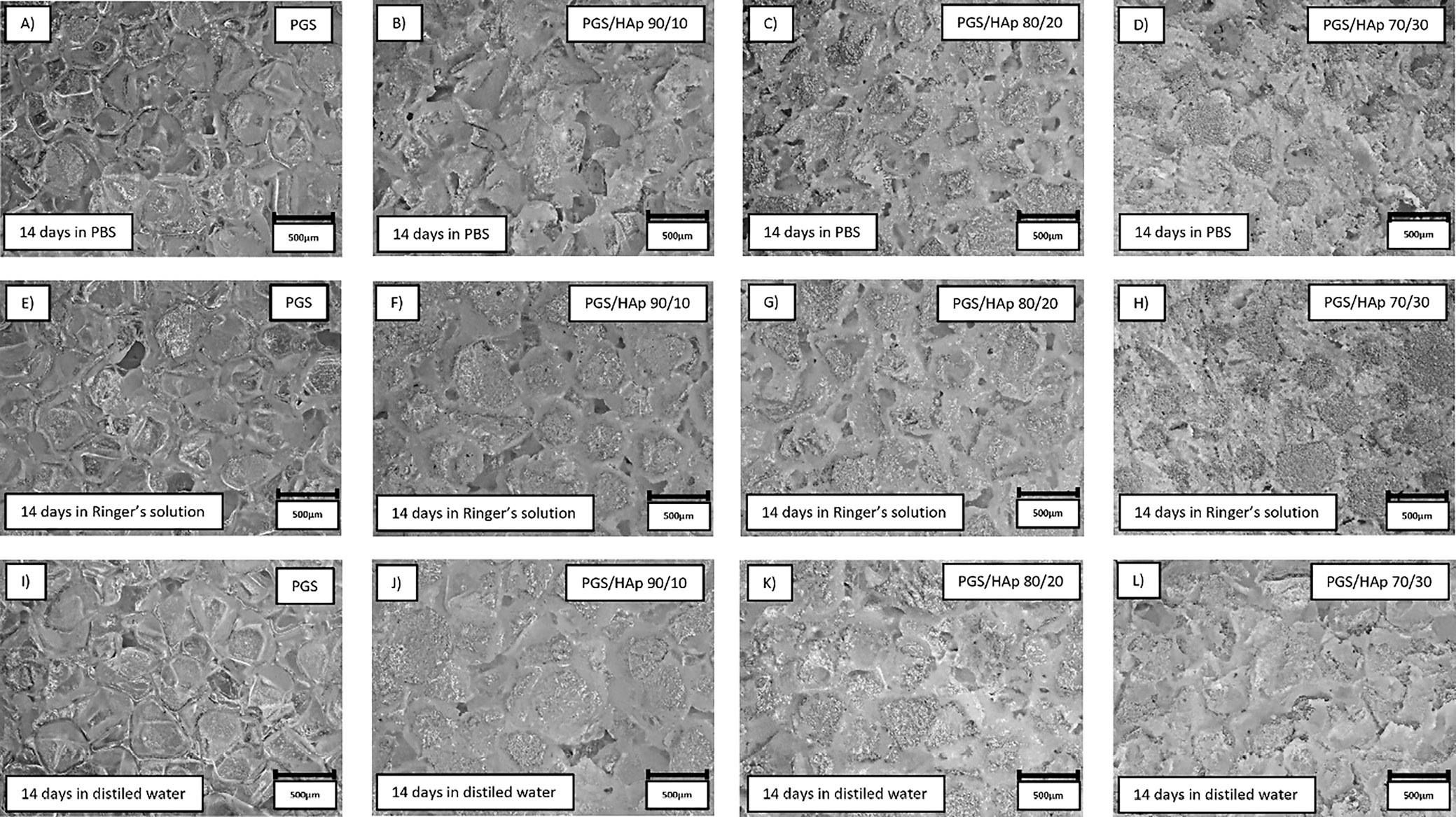
Surface all of the samples before and after 14 days of incubation in SBF, PBS, Ringer’s solution, and distilled water were registered in the form of 2D images, presented in Figure 8, Figure 9. The morphology of the top layer of the scaffolds was visualized using a 3D reconstruction of microscopic images captured on optical microscope (VHX Series Digital Microscope; Keyence). Exemplary photographs of reference samples and samples incubated in SBF and artificial saliva are presented in Figure 10.
Discussion
In artificial saliva, the greatest increased in pH values were observed among all evaluated buffers. One of the explanations of this effect may lay in the initial slightly acidic pH of the reference solution (5.08) – it may catalyze the degradation process of the scaffolds. Additionally, the ions released during the incubation might have influenced further changes in the pH values. Moreover, during incubation, partial leaching of HAp from the matrix might have occurred.34 Despite its poor solubility, the solution containing HAp might have gained additional alkaline character.35 In distilled water, a slight lowering in a pH from the reference value was noted. Changes in the pH value indicate interactions occurring at the fluid–material interface as well as the fact that the foams interact with the medium. In cases of inert (neutral) material, the pH value would remain at a constant level.
Regarding values of conductivity, such steep rise in values in distilled water might be correlated with low ionic force of water, which is disturbed by sudden introduction of new electrostatically charged beings to the solution.36 Furthermore, in all showcased experiments, using distilled water eliminated the interference of other ions in the solution with evaluated scaffold.
The swelling ability of the material is related to the penetration of the liquid medium into its interior into the free spaces of the polymer chains.37 In the case of composites, ceramic grains occupy these spaces; hence, a decrease in sorption capacity is observed as the proportion of the ceramic phase increases. However, even a low swelling capacity is a satisfactory result, as such a material can be used as a carrier for an active substance, from which the substance (e.g., a drug, protein or antibiotic) will be able to be slowly released as the liquid medium penetrates into the material.38, 39, 40
The changes in surface roughness of the scaffolds might indicate either deposition on new HAp particles or salts present in the solutions. In case of polymer scaffolds, the HAp is not present and thus the gain in mass is due to the plausible crystallization of the salts present in solutions. This hypothesis was confirmed by decrease in mass for all samples incubated in distilled water, which does not contain statistically significant concentrations of ions. The increase of the pH value during incubation in artificial saliva was the greatest among all measured solutions, which might be an indication of degradation process occurring.41 This was confirmed by loss in mass for PGS sample (approx. −5%). For specimens containing the apatite filler in artificial saliva, the mass change was positive – again most probably due to the salts present in the solution. The initial conclusion regarding crystallization of salts from incubation solutions was supported with changes in mass after subsequent rinsing in water before drying following incubation. Noticeably, the roughness of samples incubated in the distilled water overlapped with the reference values almost ideally. Moreover, the presented data indicate that the artificial saliva was the only solution for which the roughness was greater than for the reference sample. It might be yet another indication of degradation process occurring in this solution. For all other physiological solutions (SBF, PBS and Ringer’s solution), the roughness was lowered in comparison with the reference. Therefore, deposition of additional layers on top of the scaffolds might have occurred. As it is clearly visible after incubation in SBF, deposition of additional layers on the surface was observed, while in saliva, the structure appear more frayed and degraded, except for PGS/HAp 70/30 sample.
Conclusions
We investigated the initial (14 days) degradation stage of PGS scaffolds, as well as PGS doped with HAp in 5 different liquids – distilled water, SBF, PBS, Ringer’s fluid, and artificial saliva. The results obtained in SBF, PBS, Ringer’s solution, and artificial saliva provide a biologically relevant context for potential biomedical applications, as these media simulate the environment of body fluids. Water serves as a reference medium to assess baseline degradation properties. The study showed that scaffold of neat PGS showed the lowest stability, understood as weight loss during degradation (−15% after 14 days of incubation), while doped PGS showed significantly higher stability (−2% after 14 days of incubation). The effect obtained does not depend significantly on the ceramic content (similar values were obtained for systems containing 10, 20 and 30 wt% of ceramics). The greatest loss of PGS scaffold masses is particularly related to degradation in distilled water and artificial saliva. Electrical conductivity tests carried out during incubation showed no significant differences for samples incubated in SBF, PBS and Ringer’s fluid, while in the case of artificial saliva, an increase in conductivity was observed from a level of approx. 30 µS at the start of incubation to more than 40 µS after 14 days of incubation in all samples tested. Incubation conducted in distilled water led to an increase in conductivity from a level of approx. 30 µS to 80 µS for the reference sample and from approx. 40 µS to 120–150 µS for the composite scaffolds. This effect is related to the degradation of the PGS and the ingress of mineral matter from the composite into solution. The observed degradation is a beneficial process, as it will eventually be combined with the regeneration of autologous tissue, which aligns with the goals of biomedical applications where scaffold resorption supports tissue regeneration.
The conclusions for future research highlight the necessity for further investigation of long-term degradation beyond the initial 14 days, as well as the exploration of the effects of additional ceramic additives and bioactive compounds on scaffold performance. Furthermore, in vivo validations are required to confirm the behavior of the scaffold in physiological conditions and its integration with tissue. A limitation of this study is that it focuses exclusively on in vitro conditions, which may not fully capture the complexities of the in vivo environment.