Abstract
Background. One of the key challenges in tissue engineering area is the creation of biocompatible scaffolds that support cell growth and mimic the structural and mechanical properties of native tissues. Among various materials used for scaffold fabrication, composite materials based on biodegradable polymers reinforced with bioactive inorganic fillers have attracted significant attention due to their properties. One of the important problems with the preparation of composite electrospun fibers is the low filler content in the fiber.
Objectives. This study aims to select the best composition for electrospun polymer fibers in terms of potential application in tissue engineering. The effect of the viscosity of polymer solution/dispersion and filler content on the structure and properties of the fibers was determined. Morphology and filler content were compared.
Materials and methods. Series of electrospun composite fibers were fabricated from poly(ε-caprolactone) (PCL), poly(L-lactic acid) (PLLA) and hydroxyapatite (HAP), containing from 10 wt% to 40 wt% HAP. The properties of the resulting composites were studied using scanning electron microscopy (SEM), differential scanning calorimetry (DSC) and viscosimetry measurements.
Results. The addition of HAP to the polymer solution caused a significant increase in viscosity, but the results showed that it is possible to obtain composite electrospun fibers even with 40 wt% filler content. Scanning electron microscopy analysis shows randomly oriented electrospun fibers with an average diameter in the range of 3.8–8.5 μm for solution and dispersion with high viscosity (1,210–2,000 mPa·s) and significantly larger diameters (approx. 12 μm) for the PCL solution (326 mPa·s).
Conclusions. It is possible to transform the composite dispersion from biopolymers and HAP into nonwoven fabrics at up to 40 wt% filler content. Due to their unique properties, such materials are promising for application in tissue engineering.
Key words: electrospun fibers, bio-based materials, hydroxyapatite, bone regeneration
Streszczenie
Wprowadzenie. Jednym z kluczowych wyzwań w dziedzinie inżynierii tkankowej jest wytworzenie biokompatybilnych rusztowań, które wspierają wzrost komórek i naśladują strukturalne i mechaniczne właściwości natywnych tkanek. Spośród różnych materiałów wykorzystywanych do produkcji rusztowań, ze względu na swoje właściwości materiały kompozytowe oparte na biodegradowalnych polimerach wzmocnionych bioaktywnymi napełniaczami nieorganicznymi zyskały znaczną uwagę. Jednym z istotnych problemów związanych z przygotowaniem kompozytowych elektroprzędzonych włókien jest niska zawartość napełniacza we włóknie.
Cel pracy. Niniejsza praca miała na celu określenie najlepszego składu elektroprzędzonych włókien polimerowych pod względem potencjalnego zastosowania w inżynierii tkankowej. Zbadano wpływ lepkości roztworu/dyspersji polimeru i zawartości napełniacza na morfologię oraz właściwości włókien.
Materiały i metody. Wytworzono serię mat kompozytowych z polikaprolaktonu (poly(ε-caprolactone) (PCL)), kwasu polimlekowego (poly(L-lactic acid) (PLLA)) i hydroksyapatytu (HAP), zawierających od 10% do 40% wag. HAP. Właściwości otrzymanych materiałów zbadano za pomocą skaningowej mikroskopii elektronowej (scanning electron microscopy (SEM)) i skaningowej kalorymetrii różnicowej (differntial scanning calorimetry (DSC)) oraz wykonano pomiary lepkości.
Wyniki. Dodatek HAP do roztworu polimeru spowodował znaczny wzrost lepkości, ale uzyskane wyniki pokazały, że możliwe jest wytworzenie kompozytowych włókien dobrej jakości nawet przy zawartości 40% wag. napełniacza. Zdjęcia SEM przedstawiają losowo zorientowane włókna o średnicach w zakresie 3,8–8,5 μm dla roztworu i dyspersji o wysokiej lepkości (1210–2000 mPa-s) i znacznie większych średnicach (ok. 12 μm) dla roztworu PCL (326 mPa·s).
Wnioski. Możliwe jest przekształcenie dyspersji kompozytowej na bazie biopolimerów z dodatkiem HAP w włókniny o zawartości napełniacza do 40% wag. Ze względu na swoje unikalne właściwości, takie materiały są obiecujące do zastosowania w inżynierii tkankowej.
Słowa kluczowe: włókna elektroprzędzone, biomateriały, hydroksyapatyt, regeneracja tkanek kostnych
Background
Electrospinning of biodegradable polymers is a modern technique for producing fibrous structures widely used in regenerative medicine that involves forming fibers from a polymer solution using electrostatic forces generated by high-voltage electric fields. Those forces stretch liquid to the form of thin fibers, which are deposited on a collector to form matrices that may resemble the structure of natural tissues.1, 2
Among the popular polymers used in this process are polylactide,3, 4 polycaprolactone,5, 6, 7 and lactic and glycolic acid copolymers8, 9 characterized by biocompatibility and biodegradability, which allows them to be used safely in the body.10, 11 It is possible to easily modify their mechanical and surface properties, allowing them to be tailored to the specific needs of different therapies.12
Various fillers are added to polymers to enhance fiber properties and create composites with improved performance.6, 13, 14 One of the most popular fillers used in composite fibers is hydroxyapatite (HAP), naturally found in bones and teeth. Its addition increases bioactivity, stimulating osteogenesis and accelerating bone tissue regenerative processes.15 Hydroxyapatite also helps to integrate the implant into the tissue, reducing the risk of rejection and promoting the formation of new bone structures.16, 17
Nonwoven fabrics prepared with electrospinning can be used as scaffolds for tissue engineering. Because of their high surface-to-volume ratio and porous structure, these scaffolds allow efficient exchange of nutrients and metabolites, which promotes the regeneration process.18, 19 Using biopolymers reduces the risk of long-term inflammatory reactions after implantation, as the material gradually decomposes and is resorbed in a controlled way.
Objectives
The study aimed to obtain composite nonwoven fabrics made of biocompatible polymers, containing the highest possible amount of bioactive filler (HAP), which support cell growth, differentiation and new tissue formation.20, 21, 22 The unique structure of nonwoven fabrics obtained with electrospinning mimics very well the structure of real tissues, which, combined with the bioactivity of HAP, offers the possibility of their potential use in regenerative medicine.
Materials and methods
The following reagents were used: poly(ε-caprolactone) (PCL; CAPA 6800, Mw ~80,000 g/mol; Perstorp Specialty Chemicals AB, Perstorp, Sweden), Resomer L210s (Mw ~600,000 g/mol; Evonik, Essen, Germany), HAP (synthetic, 99.8%, Sigma-Aldrich, St. Louis, USA), and solvents (POCH S.A., Gliwice, Poland).
Solution/dispersion preparation
In the first step, 3 wt% solution of poly(L-lactic acid) (PLLA) in a mixture of chloroform/N,N-dimethylformamide (9/1v/v) and 15 wt% PCL solution in a mixture of chloroform/methanol (3/1v/v) were prepared. Next, the required amounts of HAP were suspended in the polymer solutions and sonicated for 30 min to obtain a homogeneous dispersion.
Electrospinning process
The electrospinning process was carried out under constant environmental parameters, at 25°C, 40% humidity and constant speed of the rotating drum collector at 300 rpm. The other process parameters were set individually for every mixture: voltage on the needle and collector (from −30.0 kV to 30.0 kV), needle-to-collector distance (100–180 mm) and the solution flow rate (1.0–6.0 mL/h; DOXA Microfluidics, Málaga, Spain).
Characterization of the polymer solutions/dispersions and electrospun mats
For viscosity measurements, a Brookfield DV1 rotational viscometer (AMETEK Brookfield, Middlesborough, USA) was used. The morphology and size of fibers were determined with scanning electron microscopy (SEM) images (Nova NanoSEM 200; FEI, Eindhoven, the Netherlands). The differential scanning calorimetry (DSC) measurements were performed with a Mettler-Toledo DSC1 system (Mettler-Toledo, Columbus, USA) under the following conditions: ~5.5 mg; N2: 60 mL/min; 10°C/min; from –80°C to 120°C (PCL samples) or 0–200°C (PLLA-based samples); thermal equilibrium: 120°C or 200°C for 5 min; then, the solutions were cooled down to −80°C or 0°C, respectively. The crystallinity degree (Xc) was calculated as (Equation 1):
(1)
where: ΔHm – measured enthalpies of melting of PCL or PLLA samples, ΔHm100% – the enthalpy of melting of fully crystalline PCL (ΔHm100% = 139 J/g))23 or PLLA (ΔHm100% = 93.7 J/g)),24 ΔHCC – measured enthalpies of cold crystallization of PLLA samples, and w – mass fraction of polymer.
Results and discussion
The effect of solution properties and process parameters on polymer and composite fiber morphology obtained with electrospinning was investigated. Especially, the viscosity of the solution has an important role in determining the range of concentrations from which continuous fibers can be obtained. The addition of dimethylformamide (DMF) to the other solvent (9/1v/v) enabled the production of defect-free and uniform fibers. A binary solvent system containing the 2nd solvent (DMF) with a higher boiling point evaporates slower from the ejected charged jet, causing the jet’s viscoelastic properties to change and therefore improving the jet’s stretching.
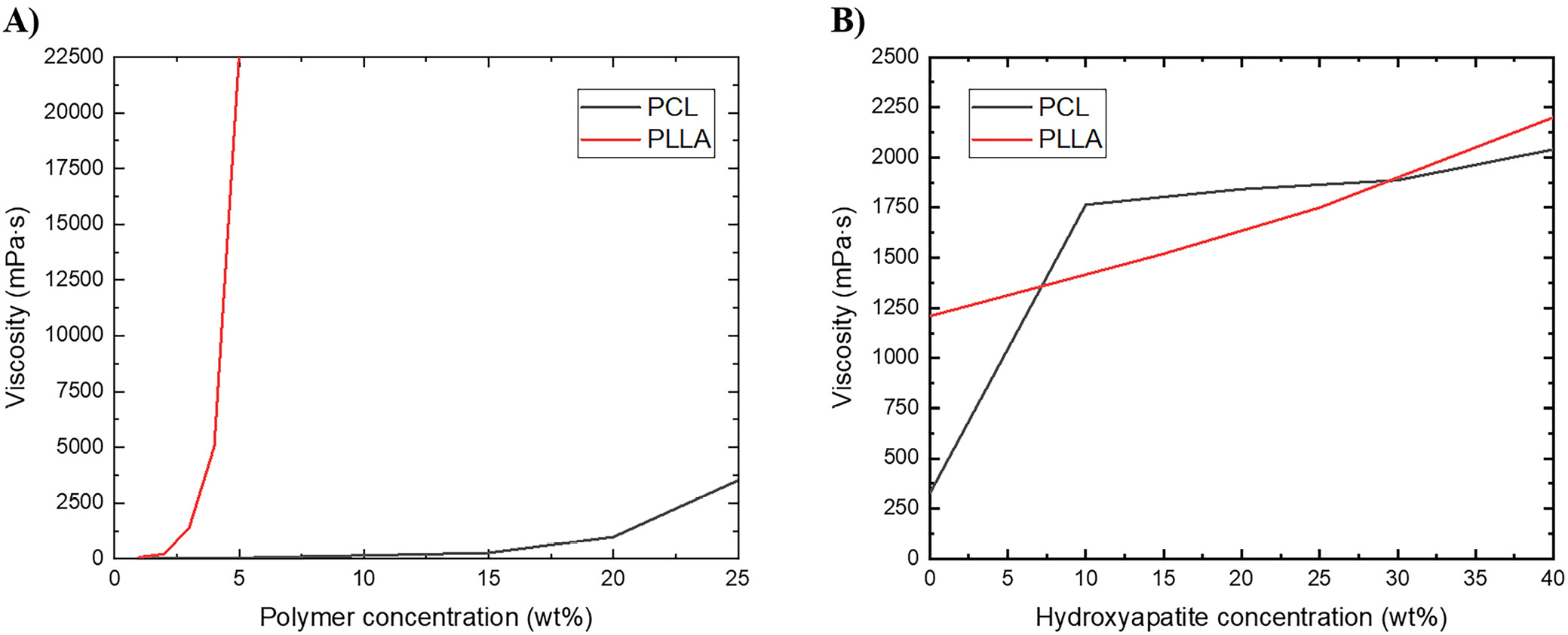
However, obtaining composite fibers with mineral filler is a challenge. One of the key parameters determining the success of the electrospinning process is the proper viscosity of the solution/suspension. Even a small addition of filler significantly increases the viscosity of the electrospun dispersion, resulting in difficulties with obtaining homogeneous fibers associated with needle plugging. For this reason, it was necessary to select new, optimal electrospinning process parameters for generating PLLA-HAP or PCL-HAP composite fibers. Each time, the voltage set at the needle is less than at the collector. However, for a pure polymer solution, this value is significantly lower. The flow rate of the electrospun solution/suspension had to be increased with increasing viscosity (from 1.0 mL/h for the pure polymer solution to 6.0 mL/h for the highest viscosity samples).
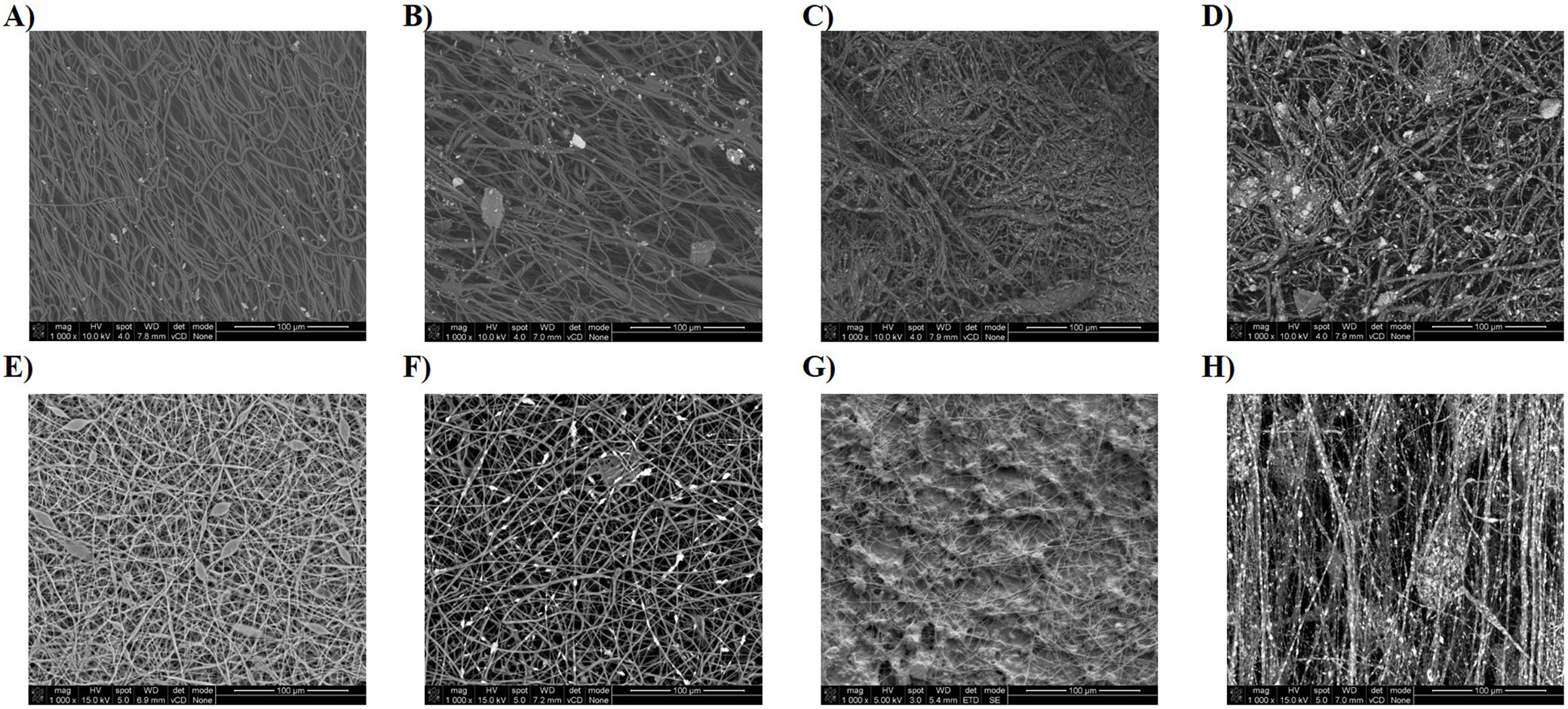
The morphologies of all electrospun fibers are shown in Figure 1. Scanning electron microscopy analysis shows randomly oriented electrospun fibers with an average diameter presented in Table 1. For most samples, uniform fibers were obtained over the entire electrospun mats. Spindle-shaped deformations were observed on some fibers formed from a solution of pure PLLA (Figure 1E). The reason for their formation may be incomplete evaporation of the solvent during the electrospinning process. The images also confirmed the incorporation of HAP particles into polymer fibers. The highest degree of fiber filling was obtained for the polylactide dispersion PLLA_HAP_60/40 at the process parameters: 15 kV, 6.0 mL/h and 180 mm. In this case, it was possible to fill the fibers with 40 wt% HAP due to the lower impact of mineral filler on the overall suspension viscosity. Up to now, it has been possible to obtain fibers with a maximum HAP content of 30 wt% relative to the polymer.14, 15, 25 Moreover, nonwoven fabricated from this dispersion was the best oriented, and HAP particles were better distributed (Figure 1H) than in other combinations. The same polymer/filler ratio was tested for the 2nd polymer (PCL); however, the viscosity increase made it impossible to carry out the electrospinning process and thus form fibers.
Despite slight differences in viscosity values for the 2 polymers, the process was substantially more efficient for electrospinning from the PLLA solution. This may be due to the difference in polymer solution concentrations. In the case of PLLA, it is already possible to have a polymer concentration 5 times lower (3 wt%) in order to obtain a solution with the viscosity necessary for the electrospinning process and produce mats of good quality, while the minimum concentration for PCL is 15 wt%.
In the case of PCL, a significant effect of HAP addition on solution viscosity was observed (addition of 10 wt% HAP increased the viscosity of 15 wt% PCL solution more than 5 times; Figure 2). For PCL with increasing HAP content, and at the same time viscosity of the system, the average fiber diameter decreases. In the case of PLLA, the effect of the presence of mineral filler on viscosity is significantly smaller. This is also reflected in the diameters of fibers obtained in the electrospinning process, which in the case of PLLA have similar diameters regardless of HAP content. From the solution with the lowest viscosity and highest polymer concentration (PCL 15 wt%, 326 mPa·s), fibers with significantly larger diameters (approx. 12 μm) were obtained. Viscosity of the remaining solutions and dispersions is in the range of 1,210–2,000 mPa·s and fibers obtained from them have average diameter in the range of 3.8–8.5 μm.
The melting and crystallization behavior of electrospun mats was investigated using DSC. As shown in Table 2, the crystallization temperature appears at approx. 116°C for pure PLLA and 30°C for pure PCL, and increases to 118°C and 32°C for PLLA and PCL composite samples, respectively. The subsequent heating curves of PLLA samples show a baseline shift and an endothermic peak located at approx. 72°C and approx. 170°C, respectively, corresponding to glass transition and melting of α/α’ crystals. Besides, an exothermic peak present in the 1st heating curve at 76–84°C can be assigned to cold crystallization. For PCL composite samples Tg is approx. −71°C and −69°C for pure polymer, while Tm is approx. 58°C.
Conclusions
This paper presents the possibility of creating bioactive polymeric composite scaffold based on 2 types of biopolymer (PCL and PLLA) and micro-sized HAP particles in varying ratios, using the electrospinning technique. It was shown that the solvent properties, especially polymer concentration and viscosity, have a significant effect on process productivity, morphology and diameter of the fibers.
Incorporation of a large amount of HAP particles into polymer fibers made them more hydrophilic, which can be useful for tissue engineering applications. These results highlight the potential of using electrospun polymer nonwovens combined with HAP in tissue engineering as materials for bone regeneration. Hydroxyapatite excellently supports bone cell growth and matrix formation. Moreover, the electrospun fiber structure can mimic the natural extracellular matrix (ECM). However, fabrication of stable and durable fibers with HAP requires precise and high control over the electrospinning process, which can be challenging.