Abstract
Background. The drug interactions with the lipid membranes are crucial in many biochemical processes. Phospholipid model membranes are often used to assess such interactions. Our team has been researching new compounds with anti-inflammatory and analgesic effects for many years. Such compounds are derivatives of the well-known non-steroidal anti-inflammatory drug (NSAID) – meloxicam (MLX). Their biological target is cyclooxygenase (COX) – a membrane protein. The NSAIDs are mainly taken orally; therefore, drug–membrane interaction is a preliminary stage in the body.
Objectives. The purpose of the present work was to investigate the ability of 2 new MLX derivatives (compound PR51 and PR52) to interact with model membranes, in comparison to known NSAIDs medicine – MLX. The differential scanning calorimetry (DSC) method was used to study those interactions. As a model membrane, bilayers obtained from a phospholipid (1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine (DPPC)) were used.
Materials and methods. Calorimetric measurements were performed using a differential scanning calorimeter DSC 214 Polyma equipped with an intracooler IC70.
Results. All examined compounds decreased the main transition temperature of DPPC in a concentration-dependent manner. The addition of studied compounds to DPPC also resulted in broadening of the transition peaks. Moreover, all examined compounds decreased the enthalpy of the DPPC main phase transition. For all DPPC gel–liquid crystalline phase transition parameters, the most pronounced effects were found for PR51 compound.
Conclusions. We have shown that the above interactions depend on the chemical structure of individual compound. All studied compounds alter biophysical properties of phospholipid bilayer.
Key words: meloxicam, DSC, model membranes, drug–membrane interaction, benzothiazine derivatives
Streszczenie
Wprowadzenie. Interakcje leków z błonami lipidowymi mają kluczowe znaczenie w wielu procesach biochemicznych. Błony modelowe fosfolipidów są często wykorzystywane do badania takich oddziaływań. Nasz zespół od wielu lat prowadzi badania nad nowymi związkami o działaniu przeciwzapalnym i przeciwbólowym; są to pochodne znanego niesteroidowego leku przeciwzapalnego (NLPZ) – meloksykamu. Ich celem biologicznym jest cyklooksygenaza (COX) – białko błonowe. NLPZ są przyjmowane głównie doustnie, dlatego interakcja lek–błona jest wstępnym etapem losu leku w organizmie.
Cel pracy. Celem niniejszej pracy było zbadanie zdolności dwóch nowych pochodnych meloksykamu (związku PR51 i PR52) w porównaniu ze znanym lekiem z grupy NLPZ – meloksykamem, do interakcji z błonami modelowymi. Do zbadania tych oddziaływań wykorzystano metodę różnicowej kalorymetrii skaningowej (DSC). Jako błonę modelową wykorzystano dwuwarstwy, otrzymane z fosfolipidu – 1,2-dipalmitoilo-sn-glicero-3-fosfatydylocholiny (DPPC).
Materiały i metody. W niniejszej pracy opisano wyniki badań kalorymetrycznych 2 nowych analogów meloksykamu (jak również samego meloksykamu) na przemiany fazowe dwuwarstw fosfolipidowych otrzymanych z DPPC. Pomiary kalorymetryczne wykonano przy użyciu różnicowego kalorymetru skaningowego DSC 214 Polyma wyposażonego w chłodnicę wewnętrzną IC70.
Wyniki. Wszystkie badane związki obniżały temperaturę głównej przemiany fazowej DPPC w sposób zależny od stężenia. Dodanie badanych związków do DPPC skutkowało również poszerzeniem pików przemiany. Ponadto wszystkie badane związki obniżyły entalpię głównej przemiany fazowej DPPC. W przypadku wszystkich parametrów przemiany fazowej ze struktury żelu w strukturę ciekło-krystaliczną DPPC najwyraźniejsze efekty stwierdzono dla związku PR51.
Wnioski. W niniejszej pracy wykorzystano metodę DSC do zbadania oddziaływań meloksykamu i jego dwóch pochodnych z dwuwarstwami fosfolipidowymi DPPC. Wykazaliśmy, że oddziaływania te zależą od budowy chemicznej poszczególnych związków. Można stwierdzić, że wszystkie badane związki zmieniają właściwości biofizyczne dwuwarstw fosfolipidowych.
Słowa kluczowe: DSC, meloksykam, pochodne benzotiazyny, oddziaływania lek–błona, modelowe błony
Background
The drug interactions with the lipid membranes is an important issue in numerous biochemical processes.1 Phospholipid model membranes are often applied as barriers which enable assessment of selected interactions.2 The polymer science focuses on the molecular structures which are developed from repeating monomer elements. A similar scheme may be observed in the phospholipids molecules, although the carbon chains in their structure are classified as hydrocarbon molecules.3
Drug interactions with natural membranes are important factors for revealing molecular mechanism of action of active pharmaceutical ingredients (APIs), as well as their pharmacokinetics.4 Some of the most commonly used drugs are painkillers, which also have anti-inflammatory effects. The typical pharmacological activity of nonsteroidal anti-inflammatory and analgesic drugs (NSAIDs) is a result of blocked activity of cyclooxygenase (COX). This enzyme is anchored in the endoplasmic reticulum (ER) and microsome membrane. It affects and enables the biosynthesis of molecules engaged in inflammatory state: prostanoids – i.a., thromboxane, as well as prostaglandins, including prostacyclin (i.e., derivatives of arachidonic acid).5 Because of the very frequent use of painkillers in many patients globally, their side effects such as gastro- and nephrotoxicity or allergic reactions are very well known; therefore, there is an urgent need to search for new, safer drugs with such effects.
Our team has been researching new compounds with anti-inflammatory and analgesic effects for many years. In chemical terms, they are derivatives of the well-known NSAID – meloxicam (MLX). In addition to the study of mechanism, toxicity and pharmacokinetics, new compounds are also tested for interactions with model membranes.
Lichtenberger et al. conducted experiments using a membrane made of mixture of phospholipid (1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine (DPPC)) and NSAIDs – diclofenac, indomethacin, naproxen, and salicylic acid derivatives were embedded into lipid matrix. The obtained results indicated that these drugs interact with non-charged phospholipids contained in the mucosa of stomach and intestines by forming complexes with them. Such an interaction promotes the gastrotoxic effects due to the reduction of the lipophilic nature of the protective mucosa and an increase of its susceptibility to damage.6
Chakraborty and Sarkar investigated the interactions of 2 oxicams with liposomes obtained from DMPC (dimyristoylphosphatidylcholine) and DMPG (dimyristoyl-L-α-phosphatidyl-DL-glycerol). The DMPC is classified as a lipid with zwitterionic properties – the polar group is relatively large, whereas DMPG with its anionic phosphoryl group has a smaller headgroup size.7 They demonstrated that partitioning of drug molecules of different dipole moment may be directed by specific properties of the membrane, including lipid spatial layout, which all may influence hydrophobicity of the lipid bilayer, even when the direct electrostatic interactions are negligible.7 Their research confirms that the interactions between drugs and the lipid bilayer need to be studied experimentally, and in silico predictions resulting from knowledge of partition coefficient value of compounds are not sufficient.
Lucio et al. showed that the NSAIDs they tested (acemetacin, indomethacin and nimesulide) influenced the phase transition temperature. Both the main phase transition and the initial phase transition temperature as well as the enthalpy value of the process in the lipid membrane were affected in this study. The strongest effect was observed for acemetacin and indomethacin, which was probably related to their good penetration of the bilayer. Moreover, it was concluded that this effect may have a negative impact to the gastrointestinal mucosa, which is associated with the occurrence of side effects of these medicines.8
The thermal effects of selected oxicam derivatives with anti-inflammatory activity, i.e., lornoxicam, MLX, piroxicam, and tenoxicam, were evaluated by Kyrikou et al. using the differential scanning calorimetry (DSC) method. The examined derivatives were immersed in the membrane bilayer based on DPPC.9 The perturbing effect of the drugs exerted on DPPC bilayers resulted in a decrease of the major phase transition temperature and in widened peak of the temperature of phase transition temperature, whereas the initial phase transition temperature vanished.
Results of all the above studies show that understanding the interaction of NSAIDs with model membranes may reveal more detailed view of mechanisms of biological activity, as well as of possible adverse effects, including the common gastrotoxicity.
Objectives
In this paper, we present the interactions of 2 new MLX derivatives with model biological membranes prepared in our laboratory (Figure 1). The aim was to investigate the ability of 2 new MLX derivatives (PR51 and PR52) to interact with artificial membranes compared to a known NSAID – MLX, using thermal analysis (namely – the DSC). As a model membrane, bilayers obtained from DPPC were used.
Materials and methods
The DPPC and Tris-EDTA buffer solution (pH 7.4) were purchased from Merck Life Science (Darmstadt, Germany), and applied as non-purified marketed components. Meloxicam was obtained from Thermo Fisher Scientific (Haverhill, USA).
PR51 and PR52 (MLX derivatives) were synthesized at our laboratory. Their purity was confirmed with elemental analysis (C, H, N) together with 1H NMR, 13C NMR, FT-IR, and high-resolution mass spectrometry (HRMS). The synthesis and analysis results of PR51 and PR52 was described elsewhere.10 The chemical structure of the tested oxicam derivatives is presented in Table 1.
The differential scanning calorimeter DSC 214 Polyma (Netzsch GmbH & Co., Selb, Germany) equipped with an Intracooler IC70 was used to perform the calorimetric measurements. They were carried out in the Laboratory of Elemental Analysis and Structural Research at the Faculty of Pharmacy of Wroclaw Medical University (Poland). The method of samples preparation for calorimetric measurements has been described previously.11 The DSC measurements were performed using the heat-flow measurement method, in a nitrogen dynamic atmosphere (25 mL/min), at a heating rate of 1°C/min (over a temperature range of 30–50°C). Measurement data were analyzed offline (Netzsch Proteus® 7.1.0 analysis software; Netzsch GmbH & Co.). The measured heat was normalized per gram of DPPC. The enthalpies of phospholipid main phase transition were stated in [J/g].
Results and Discussion
Our team used DSC method, which show how the studied compounds perturb the phospholipid thermal behavior.12, 13
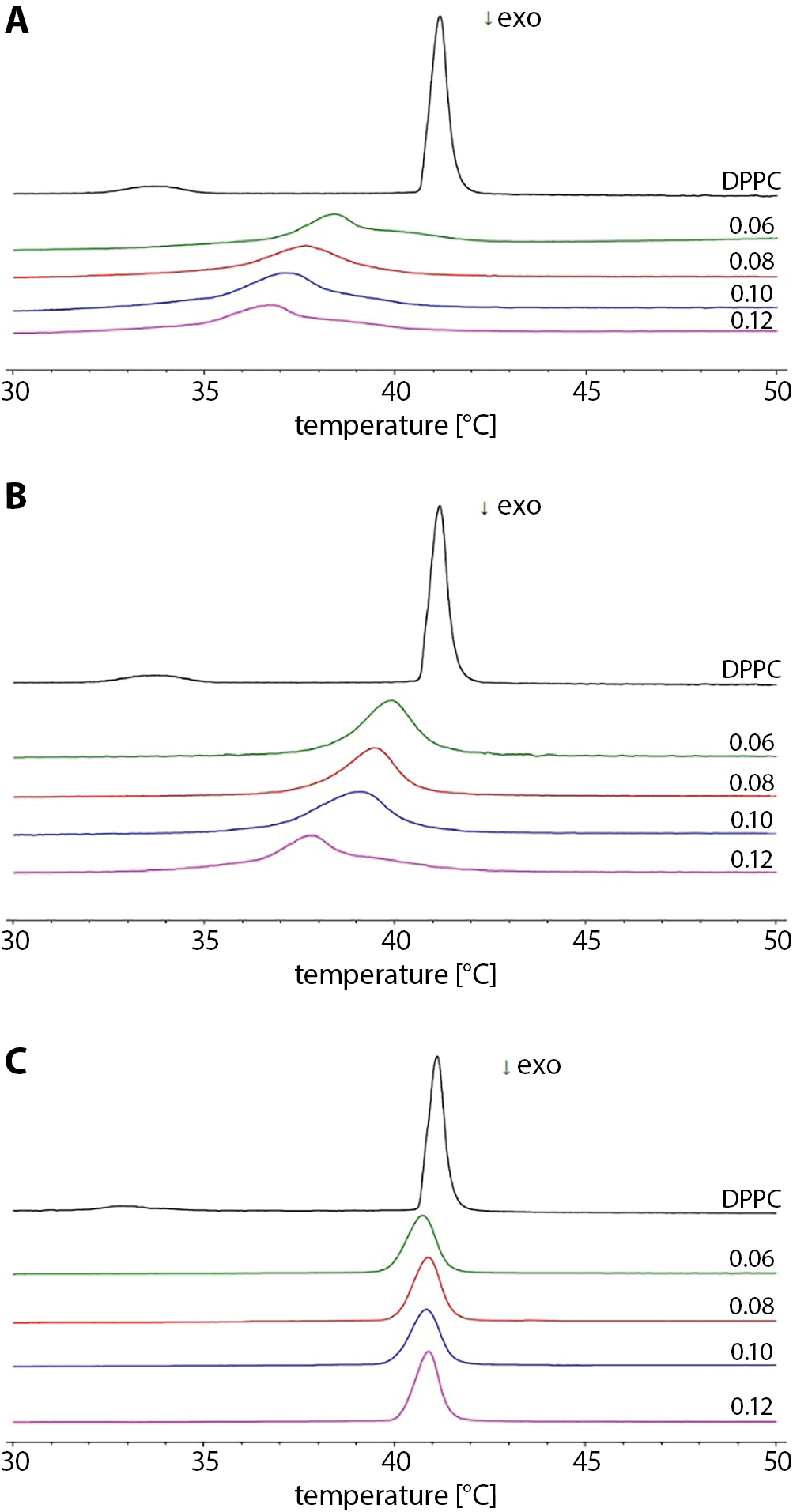
The impact of the studied additives on the phase transition profile of DPPC is presented in Figure 1. It shows the exemplary thermograms of lipid in the presence of increasing amounts of PR51, PR52 and a well-known drug from the group of NSAIDs – MLX. The perturbing effect of the studied compounds exerted on phospholipid bilayers resulted in concentration-dependent decrease of the main phase transition temperature and in widening of the peak of the phase transition temperature, whereas the pre-transition vanished (Figure 1A–C, respectively). Compounds PR51 and PR52 had a greater perturbing impact on the phase transition profile of the DPPC than MLX (Figure 1C). In DPPC membranes doped with PR51, the asymmetry of the calorimetric peaks was also observed (Figure 1A), which may suggest phase separation within a phospholipid bilayer.
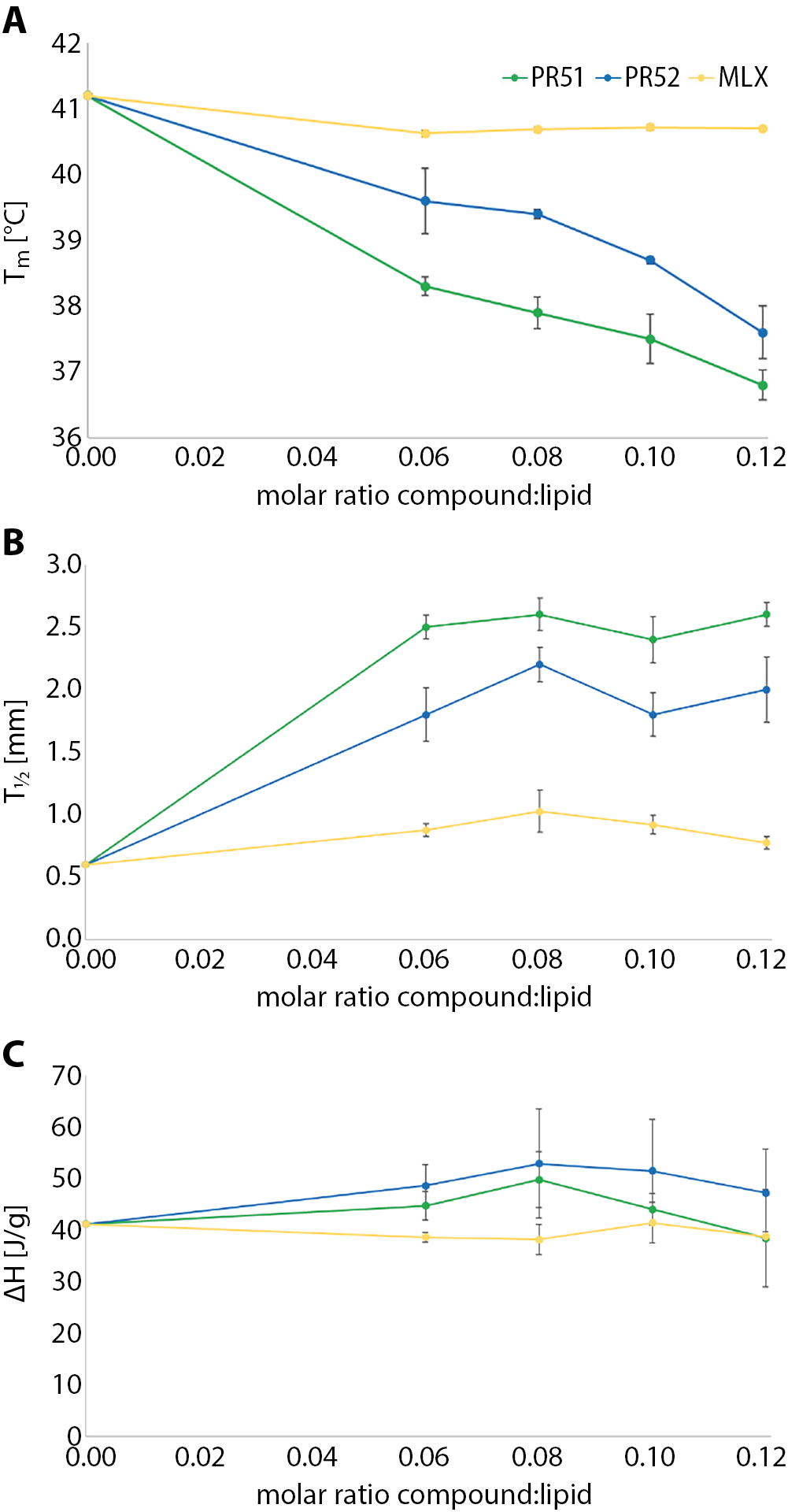
The influence of PR51, PR52 and MLX on phospholipid gel-liquid crystalline phase transition parameters – temperature (Tm), the transition peak half-height width (ΔT½) and enthalpy (ΔH) – are shown in Figure 2. The addition of studied compounds to the DPPC multi-lamellar structures increased the main transition peak half-height width (Figure 2B). It also caused concentration dependent lowering of the Tm values (Figure 2B). The addition of compound PR51 perturbed the DPPC phase transition profiles most effectively.
Our results are consistent with those of other teams that investigated the effects of NSAIDs on model phospholipid membranes.9 However, our studies showed that compounds PR51 and PR52 have a more pronounced effect on altering the phospholipid bilayer obtained from DPPC than MLX.
Limitations
In biological systems, interactions of medicines with biological membranes (which are very complex systems) may be complicated processes. For this reason, simplified models of cell membranes (e.g., phospholipid mono- and bilayers and liposomes) are used to study such processes.14 However, the use of a membrane model, which is a phospholipid bilayer made of the model phospholipid (DPPC) in a buffer solution, is a limitation of this study, because it is a simplification of the biological membrane.
Conclusions
In present work, DSC method was applied to investigate the interactions of MLX derivatives with phospholipid bilayers. The results revealed that oxicam analogues interact with model membranes obtained from DPPC. Compounds PR51 and PR52 influenced phospholipid gel–liquid crystalline phase transition parameters to a greater extent than MLX, and the effect was more pronounced for PR51, (the green curve in Figure 2), lowered the temperature of the main phase transition of DPPC the most and increased the half-width of the transition peaks (so broadened them) the most, so it also perturbed the main phase transition of the model membrane the most. That is why we may conclude that the presence of 2 chlorine substituents in MLX derivative benzene ring of side chain of the compound (PR51) seems to enhance the interaction with phospholipid bilayers, compared to the presence of only 1 fluorine substituent in the compound benzene ring of its side chain (PR52). Compound PR51 may also induce lateral DPPC phase separation in studied model membranes, probably due to the appearance of compound-rich or -poor microdomains in the lipid bilayer.