Abstract
Background. Hydrogels, containing a large amount of water and exhibiting high biocompatibility, can improve the rheological properties of formulations and adhere well to the application site. In Poland, only 1 hydrogel substrate is currently approved for pharmaceutical compounding: Celugel, based on hydroxyethyl cellulose (HEC).
Objectives. The aim of this study was to investigate how the variation in the raw material composition of Celugel-based hydrogels affects their osmotic pressure values and selected rheological properties.
Materials and methods. Ten gel formulations were prepared using a commercial Celugel as the base, with varying percentages of added water, alongside a consistent 5 wt% addition of sucrose. The research methods employed include osmotic pressure, dynamic viscosity, pH measurement, and surface tension using the du Noüy ring tensiometer.
Results. The composition of the formulation has a significant impact on the osmotic pressure. Nearly all of the hydrogels exhibited hyperosmotic characteristics relative to living tissues, with measured osmotic pressure values ranging from 160 mOsm/kg H2O to 1,480 mOsm/kg H2O. As anticipated, the viscosity of the formulations increased proportionally with the growing concentration of Celugel ranging from 2.19 mPa·s to 562.87 mPa·s.
Conclusions. The composition of Celugel significantly influences its rheological properties and osmotic pressure values, with the concentration of the gelling agent being the most impactful factor. The results suggest that Celugel is suitable for use in formulations intended for nasal administration.
Keywords: osmotic pressure, hydrogel, Celugel, hydroxyethylcellulose
Streszczenie
Wprowadzenie. Hydrożele, zawierające dużą ilość wody i charakteryzujące się wysoką biokompatybilnością, mogą poprawiać właściwości reologiczne formulacji i dobrze przylegać do miejsca aplikacji. W Polsce obecnie tylko jedno podłoże hydrożelowe jest zatwierdzone do receptury aptecznej: Celugel, oparty na hydroksyetylocelulozie.
Cel pracy. Celem badań było zbadanie, jak zmiana składu surowcowego hydrożeli na bazie Celugelu wpływa na wartości ciśnienia osmotycznego oraz wybrane właściwości reologiczne.
Materiał i metody. Przygotowano dziesięć formulacji żelowych, używając komercyjnego Celugelu jako bazy, z różnymi procentowymi dodatkami wody oraz stałym dodatkiem 5 wt.% sacharozy. W zastosowanych metodach badawczych uwzględniono pomiary ciśnienia osmotycznego, lepkości dynamicznej, pH oraz napięcia powierzchniowego za pomocą tensjometru pierścieniowego du Noüy.
Wyniki. Skład formulacji ma istotny wpływ na ciśnienie osmotyczne. Niemal wszystkie hydrożele wykazywały właściwości hipertoniczne w odniesieniu do tkanek żywych, a wartości ciśnienia osmotycznego wynosiły od 160 mOsm/kg H2O do 1480 mOsm/kg H2O. Zgodnie z oczekiwaniami, lepkość formulacji wzrastała proporcjonalnie do rosnącego stężenia Celugelu, w zakresie od 2,19 mPa·s do 562,87 mPa·s.
Wnioski. Skład Celugelu znacząco wpływa na jego właściwości reologiczne i wartości ciśnienia osmotycznego, przy czym największy wpływ ma stężenie substancji żelującej. Uzyskane wyniki sugerują, że Celugel nadaje się do stosowania w formulacjach przeznaczonych do podania donosowego.
Słowa kluczowe: hydroksyetyloceluloza, hydrożel, ciśnienie osmotyczne, Celugel
Background
Ointments are semi-solid pharmaceutical formulations intended for application on the skin or mucous membranes, functioning as emollients, protective agents or, when combined with active ingredients, as therapeutic agents. These preparations also contain excipients that provide essential organoleptic properties, rheological behavior and stability.1
According to the 12th edition of the Polish Pharmacopoeia,2 ointment bases are classified into 5 main categories, including gels. A significant subgroup within gels is hydrogels, distinguished by a 3-dimensional network structure that retains large amounts of water within a swollen polymer matrix.3 This structure closely resembles that of living tissues, providing hydrogels with superior biocompatibility. As a result, hydrogels are extensively used in medical and pharmaceutical applications such as drug delivery systems, wound dressings, tissue-engineering scaffolds, and contact lenses.4
In Poland, Celugel is an authorized hydrogel base composed of hydroxyethyl cellulose (HEC) and glycerol, preserved with sorbic acid and its potassium salt. Due to its beneficial characteristics, such as easy rinsability and high mucoadhesive capacity, Celugel is widely applied in formulations for the skin and mucous membranes, including nasal preparations. It offers a practical alternative to traditional glycerol-based formulations, which often have the disadvantage of flowing away from the application site.5
Osmotic pressure is a crucial factor influencing the local effects and effectiveness of various formulations, including those applied nasally.6 While isotonic formulations are generally preferred, especially for prolonged use, non-isotonic formulations may be acceptable in specific cases. Furthermore, osmotic pressure can drive the release of active ingredients from certain drug delivery systems, enhancing their therapeutic impact.7, 8
Objectives
The aim of this study was to investigate how variations in the composition of Celugel-based hydrogels affect osmotic pressure values and selected rheological properties.
Materials and methods
Celugel (Actifarm, Poland), hydroxyethyl cellulose with high viscosity and a molecular weight of approximately 300,000 Da (Pol-Aura, Poland) and sucrose (Pol-Aura, Poland) were used in the studies. Deionized water, used in all experiments, was obtained through an ion-exchange column in accordance with the requirements for purified water described in Polish Pharmacopoeia.9
Preparation of gels was based on Celugel with variable water content and a 5 wt% sucrose addition. A measured amount of Celugel base and water was combined in a vessel, followed by the addition of micronized sucrose, prepared in advance using a mortar. The mixture was stirred until a homogeneous gel was obtained. The specific compositions of these formulations are shown in Table 1, with all gels stored at 2–8°C.
Osmotic pressure measurements were performed, and subsequent rheological analyses included assessments of dynamic viscosity, surface tension and pH. Osmotic pressure measurements of the gels were conducted using the Marcel OS 3000 osmometer (Marcel S.A., Zielonka, Poland), an automated device enabling precise, rapid osmotic analysis based on freezing-point depression. The pH was determined conductometrically using the Elmetron CPC-505 (Elmetron, Zabrze, Poland) in conjunction with the Elmetron ERH-11S pH electrode.
Dynamic viscosity was measured with the Brookfield RVDV 3+ rotational rheometer (Ametek, Middleborough, USA), which operates by detecting the torque required to overcome the resistance exerted by the sample’s viscosity. Measurements employed 2 cone-and-plate setups: cone CP40 (0.8° angle and 4.8 cm diameter) operated at 5 rpm to generate a shear rate of 37.5 s–1, while cone CP51 (1.565° angle, 2.3–2.4 cm diameter) operated at 100 rpm with shear rates of 384 s–1, 750 s–1 and 1500 s–1, measured for 10 s at a controlled temperature of 24°C.
Surface tension was assessed using the ring method on the D-MT1A tensiometer (SITA Messtechnik GmbH, Dresden, Germany), supported by D-MT1A.exe software (Polon-Izot Sp. z o.o., Warsaw, Poland) for precise analysis.
These methods provided comprehensive data on the physicochemical properties of the gels, contributing valuable insight into their suitability for various pharmaceutical and therapeutic applications.
Results
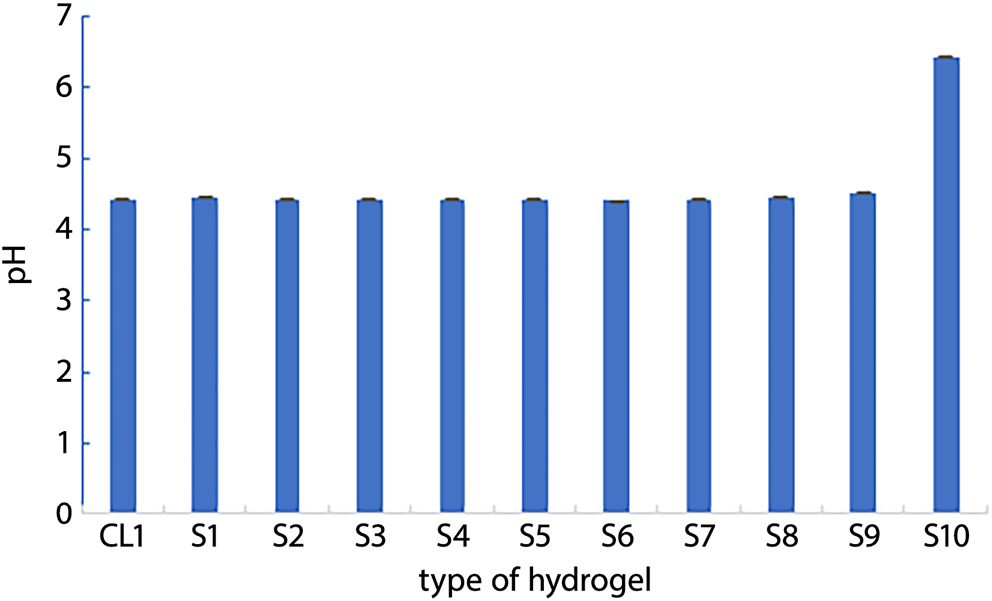
The osmotic pressure measurements for the CL1 and S1–S10 hydrogels ranged from 160 mOsm/kg H2O to 1,480 mOsm/kg H2O. Virtually all hydrogels were found to be hyperosmotic relative to living tissues, with the exception of formulation S10, which exhibited osmotic pressure values below physiological levels. The exact results of the osmotic pressure measurements are shown in Table 2. Values of pH were determined for examined (CL1 and S1–S10), The pH of gels S1–S9 ranged from 4.4 to 4.5, while a value of 6.4 was obtained for the 5 wt% aqueous sucrose solution (S10). The pH for CL1 was measured at 4.4. All results are presented in Figure 1.
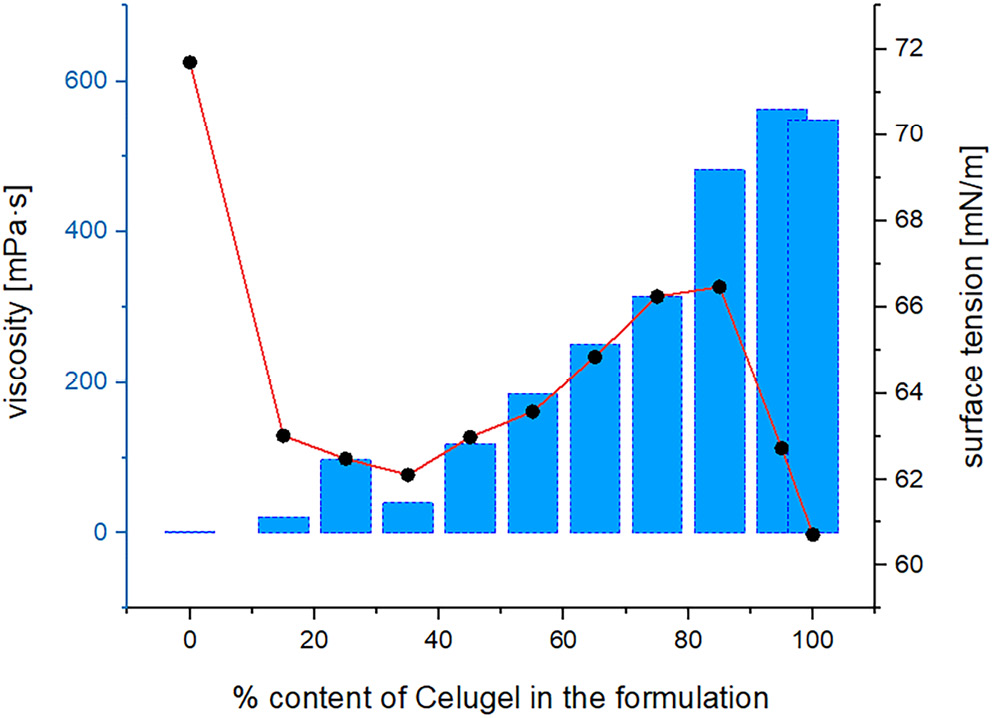
Viscosity measurements were conducted for Celugel and samples S1–S10. The mean viscosity value for Celugel was recorded at 548.57 mPa·s. For formulation S1, a slightly higher viscosity of 562.44 mPa·s was observed. Subsequent samples exhibited a decreasing trend in viscosity with a reduction in the percentage of the finished substrate within the formulation. Notably, hydrogel S8 displayed a viscosity value that exceeded those of hydrogels S7 and S9.
Surface tension values were also measured for Celugel and the S1–S10 formulations derived from it. The surface tension for Celugel alone was determined to be 60.71 mN/m, while for formulation S1, it was 62.73 mN/m. Surface tension values for hydrogels S2–S9 demonstrated a decreasing trend, ranging from 66.47 mN/m to 62.83 mN/m. Conversely, formulation S10 exhibited a surface tension value of 71.68 mN/m.
Discussion
We demonstrated the varied impacts of hydrogel formulation composition, specifically those based on Celugel, on osmotic pressure and selected rheological properties. Hydrogels were prepared with commercial Celugel, differing in water content and supplemented with a 5 wt% sucrose concentration.
Results reveal a marked influence of formulation composition on osmotic pressure. Nearly all hydrogels showed hyperosmotic values compared to physiological levels, which are typically in the range of range 275–295 mOsm/kg H2O; however, formulation S10 exhibited osmotic pressure below physiological thresholds.10, 11, 12 Commercial nasal preparations vary widely in osmotic pressure, from approx. 300 mOsm/kg H2O to as high as 700 mOsm/kg H2O. Hyperosmotic solutions may induce nasal mucosal irritation, triggering an increase in watery secretion, thereby reducing congestion and swelling of nasal tissue. Nonetheless, prolonged exposure to hypertonic formulations may damage the mucosal membrane, leading to discomfort, burning sensation or irritation, indicating limited suitability for long-term use.7, 13
In formulations S1–S10, an incremental rise in osmotic pressure was observed in correlation with increased Celugel concentration. Sucrose, given its water solubility, also contributes osmotic activity, thereby elevating the osmotic pressure within the gel matrix.14
The pH values for formulations S1–S9 demonstrated notable stability, with a maximum variance of 0.9 units. Formulation S10, a 5 wt% sucrose solution, had a slightly acidic pH of 6.4, likely due to microbial degradation of sucrose into acidic byproducts, as this formulation lacked a preservative.
The pH level of a formulation significantly affects the absorption of active ingredients through the nasal mucosa. For nasal applications, a pH range of 4.5–6.5 is generally optimal, enhancing the stability of light-sensitive compounds, supporting lysozyme activity for microbial control, and inhibiting viral replication. Although most studied formulations displayed pH values slightly above the recommended range, their potential application remains viable.15
Viscosity measurements confirmed an expected increase in viscosity with higher Celugel content, supporting the suitability of Celugel as a viscosity-enhancing agent in formulations, including nasal drops. Results also affirm the low viscosity of the base itself, which declines further upon dilution.
Surface tension in the hydrogels was measured to be lower than that of water. Hydroxyethyl cellulose, classified as a non-ionic amphiphilic molecule, decreases surface tension by disrupting the hydrogen bonding within water molecules that are bound within the hydrogel matrix.16
Analysis of viscosity and surface tension values revealed a shift in trends beginning with formulation S7, as depicted in Figure 2. This is likely due to reaching or nearing the critical micelle concentration (CMC) of HEC, which exhibits surfactant properties. At the CMC, the surface tension reaches a minimum, after which further concentration increases lead to a rise in surface tension, likely due to greater cohesion among polymer chains. The observed micelle formation suggests that beyond CMC, intermolecular forces within the polymer network intensify, impacting both viscosity and surface properties.17, 18
Conclusions
These findings provide insight into the physicochemical properties and potential therapeutic applications of Celugel-based hydrogels, informing their formulation for targeted biomedical applications. Based on them, several key conclusions can be drawn regarding Celugel-based hydrogel formulations. The formulation’s composition plays a crucial role in determining its rheological properties, which directly impacts its behavior and stability. Among the various components, the concentration of the gelling agent stands out as the primary factor affecting these properties, indicating its central role in defining the overall characteristics of the hydrogel. Furthermore, the results confirm that Celugel is a suitable candidate for formulations intended for nasal administration, suggesting its potential for effective use in targeted delivery systems through this route.