Abstract
The spinal cord is one of the most important part of the human nervous system and great importance is placed on developing the best treatment for its damage. 3D bio-printing technology, and the fabrication of special scaffolds using it, is a potential solution for regenerating damage in spinal cord injuries (SCIs). Bio-printing can be divided into indirect and direct bio-printing, while among the bio-printing methods, inkjet bio-printing, fused deposition modeling (FDM), extrusion bio-printing, or light-assisted bio-printing can be distinguished. The last group can be in turn divided into several separate techniques such as digital light processing (DLP), stereolithography (SLA) and laser-assisted bio-printing (LAB). While bio-printing technology for the treatment of SCI is in the early stages of research, several successful trials have already been performed, where the use of such scaffolds has resulted in at least partial restoration of autonomic nervous system function in patients with chronic and acute SCI.
Key words: central nervous system, spinal cord, bio-printing
Streszczenie
Rdzeń kręgowy jest jedną z najważniejszych części ludzkiego układu nerwowego. Z tego powodu przywiązuje się dużą wagę do opracowania najlepszego leczenia towarzyszących mu uszkodzeń. Technologia biodruku 3D i wytwarzanie specjalnych rusztowań z jego pomocą jest potencjalnym rozwiązaniem w zakresie regeneracji uszkodzeń w urazach rdzenia kręgowego. Głównym podziałem typów biodruku jest podział na biodruk pośredni i bezpośredni, podczas gdy wśród metod biodruku można wyróżnić biodruk atramentowy, modelowanie osadzania topionego materiału (FDM), biodrukowanie ekstruzyjne lub biodrukowanie wspomagane światłem. Ostatnią grupę można z kolei podzielić na kilka odrębnych technik, takich jak cyfrowe przetwarzanie światłem (DLP), stereolitografię (SLA) i biodrukowanie wspomagane laserowo (LAB). Chociaż technologia biodruku w leczeniu urazów rdzenia kręgowego jest na wczesnym etapie badań, przeprowadzono już kilka udanych prób, w których wykorzystanie takich rusztowań doprowadziło do przynajmniej częściowego przywrócenia funkcji autonomicznego układu nerwowego u pacjentów z przewlekłym i ostrym urazem rdzenia kręgowego.
Słowa kluczowe: rdzeń kręgowy, centralny układ nerwowy, biodrukowanie
Introduction
The spinal cord consists of externally located white matter and internally located gray matter. These are clusters of nervous tissue, which together with the brain form a system called central nervous system (CNS). The CNS is responsible for the body’s basic vital functions. It has low capacity to replace and renew neurons after damage or disease, which is the reason why degeneration in the structure of the CNS caused by disease or physical damage often leads to the loss of nerve cells, axons and glial support.1, 2, 3, 4, 5 Spinal cord injury (SCI) is considered one of the greatest challenges among CNS disorders, and the serious complications and high incidence of paraplegia caused by SCI is a growing concern for both affected individuals and their families. It also poses a significant burden to the whole society.6 Pathologically, SCI is caused by a primary injury and a series of secondary injuries. Primary injuries are mainly acute injuries caused by mechanical forces, such as spinal disc extrusion and dislocation, including damage to neurons and glial cells in the relevant segments, leading to ruptures in blood vessels.7, 8 Secondary injuries include local edema, disruption of ion homeostasis, ischemia, intense inflammatory response, and excess free radicals.9
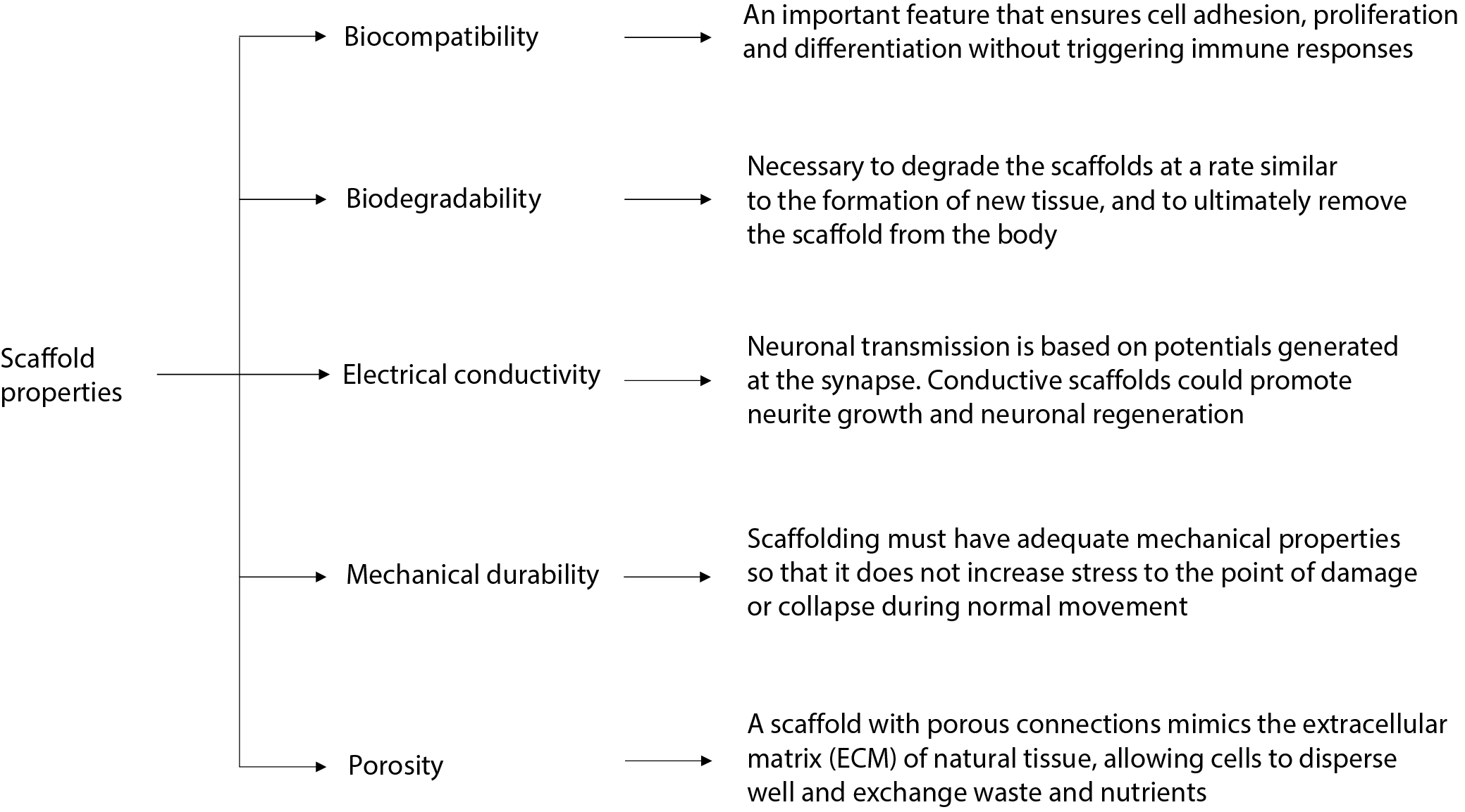
Currently, clinical treatment can be divided into surgical and non-surgical. One example of non-operative treatment is the use of high doses of methylprednisolone (MP), which is a corticosteroid that inhibits lipid peroxidation and is used to reduce the formation of secondary injuries.10, 11 However, the use of MP is limited due to a number of side effects, including increased risk of urinary tract infections, respiratory tract infections, wounds, sepsis, and pneumonia.12 In terms of surgical treatment, the most relevant methods are decompression and stiffening of the injured site. The primary goal of SCI treatment is to remove the effect of compression factors and restore spinal stability. The aforementioned methods make a small contribution to the treatment of SCIs; however, all of these methods of clinical treatment can only remove or reduce the action of the factors causing the injury, but do not enable functional regeneration of the damaged nerve. On this basis, it can be concluded that the repair is incomplete.13 Regeneration of the nervous system involves the repair and re-generation of nerve tissue cells and nerve connections. Tissue engineering involving direct replacement of nerve cells and/or repair of connections through cell transplantation, biochemical molecular signaling and targeting using so-called “scaffolds” is used for this purpose.14 The ideal scaffold for neural tissue engineering should meet several important criteria, as shown in Figure 1.15, 16
Techniques such as melt molding, gas foaming, electrospinning, and phase separation have been used in the production of scaffolds made out of synthetic and natural polymers.17 The disadvantage of techniques mentioned above is the inability to precisely control and adjust the shape of the scaffolds, the configuration of internal channels and the size of the pores in the scaffold. Additionally, these techniques do not allow for the production of scaffolds using cells due to manufacturing conditions being unfavorable for cells survival. In recent years, 3D bio-printing has emerged as a solution to these problems, attracting attention due to its ease of controlling shapes and dimensions of scaffolds and creating frameworks for cells.18
The method known as additive manufacturing (AM) is used in printing cells, growth factors and biomaterials in layers. Moreover, it allows for the creation of biological structures that exhibit properties similar to organs and mimic natural tissue.19 For this reason, 3D bio-printing has become a promising and effective strategy for repairing SCI, as AM can easily produce scaffolds for cells while selecting their appropriate dimensions. However, there are also significant challenges associated with 3D bio-printing, such as cumbersome handling, insufficient printability, low cell viability during printing, and minimal cell–material interaction.
In this work, a review and discussion of various available 3D printing methods as well as their theoretical and practical applications in the treatment of various spinal cord injuries was conducted.
3D bio-printing: Theoretical basis and examples of application
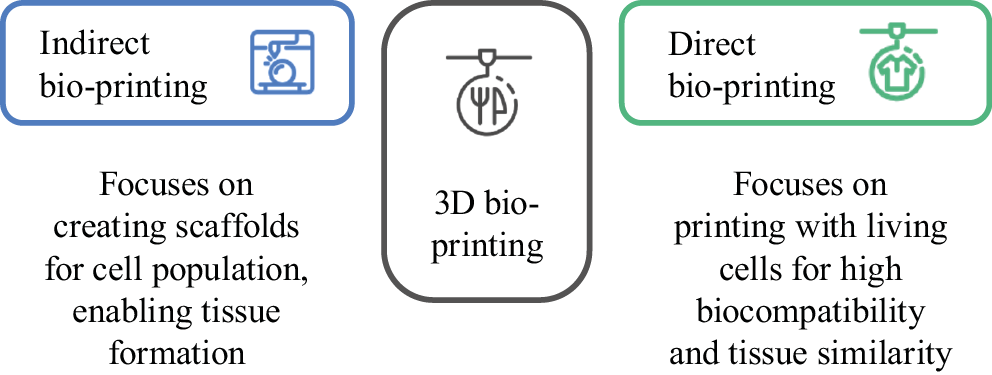
Based on the data from scientific literature, one of the basic divisions of 3D bio-printing is that into indirect bio-printing, which aims to produce scaffolds and other types of frameworks that finally can be populated by cells, and direct bio-printing, which allows for the production of structures using biological material with living cells, which in turn ensures greater similarity to naturally formed tissues and increased biocompatibility. This division in bio-printing is presented in Figure 2.
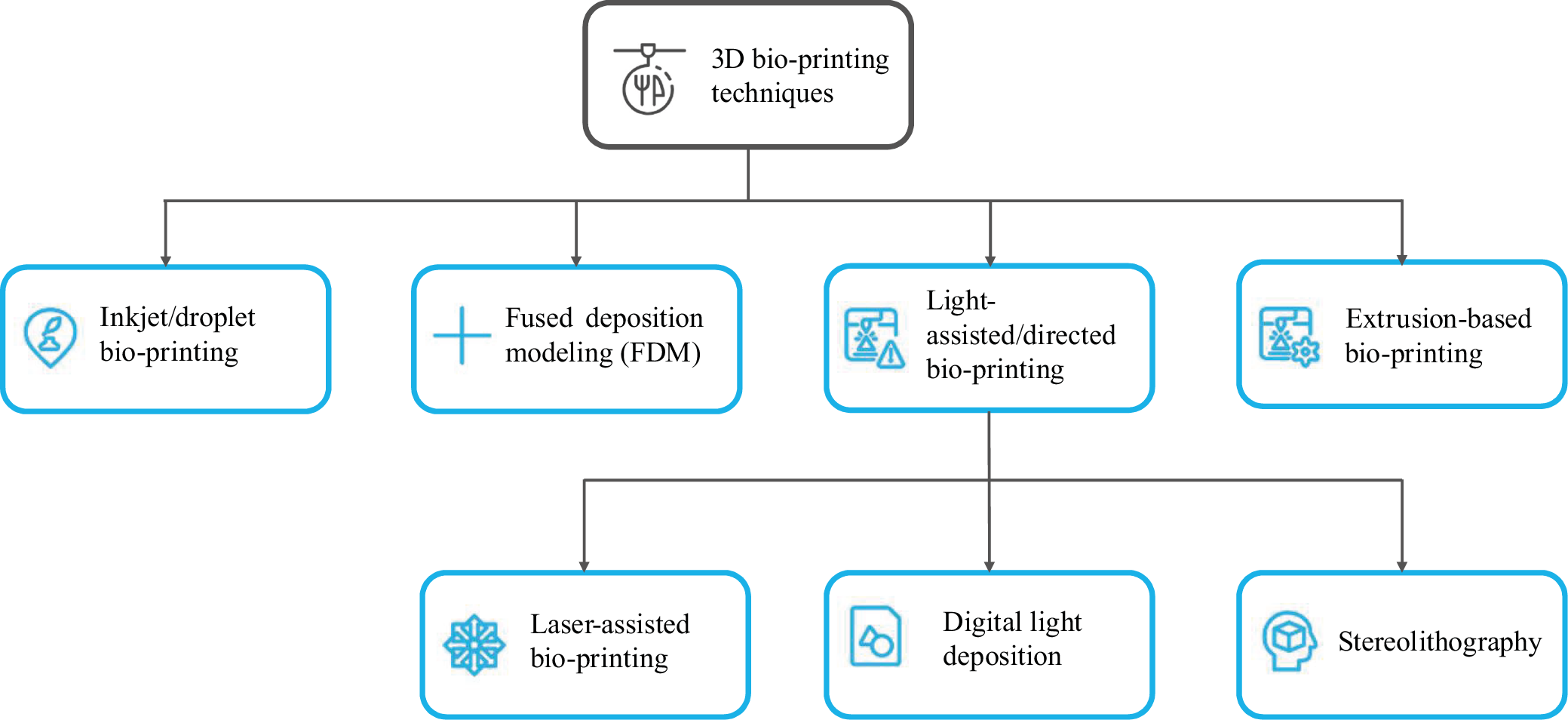
Among the 3D bio-printing methods, many types can be distinguished; however, in order to facilitate classification and for better understanding, the following division was applied: inkjet/droplet bio-printing, fused deposition modeling (FDM), light assisted/directed bio-printing, and extrusion-based bio-printing. This division has been illustrated in Figure 3.
Inkjet/droplet bio-printing
3D inkjet printing technology has been adapted from the 2D printing system, where in this method droplets of the used liquid are deposited on special platform in a “layer by layer” manner, enabling rapid production of a complete structure with micrometer resolution.20, 21 This method can be further differentiated into thermal and piezoelectric.
In thermal printers, the print head is heated to temperatures between 200°C and 300°C, which leads to the formation of pressure pulses that in turn push the droplets out of the nozzle.20 Furthermore, several studies have shown that local heating to temperatures 200°C and 300°C affects neither the stability of biological molecules nor the viability and function of tissues after printing.21 This method is characterized by high printing speed and the ability to print tissues ranging in size from 20 µm to 100 µm. Additionally, it shows potential for printing in picoliter (pL) volumes to achieve better resolution and accuracy.
In the piezoelectric method, piezoelectric actuators located in the print head are used to generate droplets, which are stimulated by applying voltage. The speed and size of the ejected droplets can be controlled by factors such as timing, pulse frequency and actuator amplitude.22 Additionally, the piezoelectric method causes less cell damage, resulting in a higher survival rate compared to the thermal method.23, 24
Common drawbacks of inkjet bio-printing are poor mechanical properties of the printed structures and their low durability. It happens because this technique is only able to distribute bio-ink with a viscosity not exceeding 10 MPa/s.23, 25 Another limitations of 3D inkjet printers are small nozzle size and flow rate, which limit the volume of a single droplet to below 10 pL. To maximize the probability that each droplet will contain a cell, cells must be seeded at a high concentration (above 5·106 cells/mL).25
Fused deposition modeling
This technique uses thermoplastic polymer filament to produce 3D structures. Depending on the polymer used, the filament is heated in a nozzle to reach semi-liquid state and in this form is extruded onto platform following a computer-designed model.26 Since this method uses high temperatures, cells are applied and cultured on the structures only after the printing process is completed.27, 28 The types of filaments used in this methods and the required temperatures are presented in Table 1.29
The use of thermoplastic polymer fibers in this method is linked to its greatest advantages – low cost and high production speed. Considering the Young’s modulus of a given polymeric biomaterial, polyetheretherketone (PEEK) appears to be the most promising. Additionally, since the FDM method does not require any additional solvents or materials, it provides convenience in terms of material handling and allows for continuous production without need to change raw materials.25 This method also has its drawbacks, such as poor mechanical properties – especially if inter-layer defects occur during printing – and poor surface properties.30
Light-assisted/directed bio-printing
Light-assisted bio-printing is a group of techniques that include laser-assisted bio-printing (LAB), digital light processing (DLP) and stereolithography (SLA). These are nozzle-free printing methods, so the complexity of the structure does not affect the printing time. Moreover, tissues obtained using this technique exhibit good biocompatibility and cell viability.31, 32
Laser-assisted bio-printing
Currently, LAB technology is based on 2 techniques – laser direct writing (LDW) and laser-induced transfer (LIFT).33 It consists of 3 main elements: a pulsed laser, a ribbon and a receiving substrate. After the laser beam is emitted, it is absorbed by the metal containing the ribbon, e.g., gold (Au) or titanium (Ti). Then, the biomaterial suspended on the ribbon evaporates under the influence of the laser, creating high-pressure bubbles that eventually settle on the receiving substrate, creating the appropriate biological pattern/scheme.34 This method is not as popular as the others described in this paper, but some researchers use it in tissue engineering. It can be attributable to the wide range of viscosities that the bio-ink can have (1–300 MPa/s), the high accuracy of printing scaffolds (accuracy 10 µm) and the possibility of obtaining a resolution of 1 cell per drop of bio-ink.23, 32, 35 Among the disadvantages both of this method one can distinguish a very long printing time and a low flow rate, which is caused by the high resolution.36, 37
Additionally, in this method, it is not possible to print simultaneously using multiple types of cells and materials, which results in a laborious and cumbersome process. A decrease in cell viability in this method below 85% has also been observed, which may be caused by thermal damage of the cells associated with the use of the laser.36
Digital light processing
Digital light processing (DLP) relies on the polymerization of light-sensitive polymers using precisely controlled light emitted from a special digital micromirror device (DMD).32 The 3D structure is obtained by moving the working platform from bottom to top. First, the working platform is immersed in the liquid, after which a 2D image layer is created on the platform. Then, the platform is moved upwards by the distance of the created layer and the process is repeated. As a result of this process, the 3D structure is created layer by layer.38 Compared to other methods, this one requires the most preparation; however, the process of creating the structure itself is fast and accurate. An example of the use of DLP is the work of Yu et al.,39 involving the printing of structures based on decellularized extracellular matrix (dCEM) with a size of only 30 µm. Using this method, complex, hierarchically branched geometries are created in a matter of seconds39 because, instead of loading the material into the cartridge, the working platform is immersed in a tank with fluid, the printing speed itself is extremely high and the structure is created layer by layer. The resulting product can more effectively simulate the biological structure of the spinal cord compared to other methods. Despite this, DLP is a relatively new technology and the appropriate gels and material used require further research, which can contribute to even greater improvement of this method compared to others.
Stereolithography
Unlike the DLP method, stereolitography (SLA) uses laser reticulation with point or line scanning, while the rest of the procedure is based on the same principle as DLP. This method has no limitations regarding cell viscosity and also allows for printing tissue structures with a resolution of approx. 100 µm.31, 40
Depending on the photoinitiator used, this method typically requires the use of visible or ultra-visible light to create covalent bonds in the bio-ink. However, in recent years, there has been a shift away from the use of ultra-visible light due to its harmful effects on cell DNA and the risk of skin cancer. Therefore, researchers are focusing primarily on visible light photopolymerization.41 In a study conducted by Wang et al.42 involving a stereolithography-based bio-printing system using visible light-crosslinked bio-inks and a commercial projector with a simple water filter, it was proven that the use of visible light as a crosslinking agent enables the printing of hydrogels with a resolution of up to 50 µm and maintains cell viability at 85%.42
Sakai et al.,43 in a similar study also involving the use of stereolithography based on visible light, to create alginate hydrogels with phenolic hydroxyl groups (Alg-Ph), obtained cell viability of about 95%.43 Despite the advantages of the SLA method, such as no limit on cell viscosity and print resolution, the mechanical limitations of this method result in a significantly slower printing process compared to other methods.44
Extrusion-based bio-printing
Of all the described bio-printing methods, extrusion-based printing is the most prevalent and well-developed technique.45 In this method, a mixture of cells and hydrogels is “extruded” through micro-nozzles or needles onto a substrate to print a 3D structure. The micro-extruder, following instructions from the CAD-CAM system, lays down the material on the substrate in the form of beads. The beads are pre-arranged in the X-Y plane/axes, after which the “extruded” head is moved along the Z axis to create a complex 3D structure.23
The most important variations of this method include methods with pneumatic and mechanical (piston and screw) distribution systems. Piston-driven systems provide better control over bio-ink flow, while screw systems allow for more precise spatial control and are useful when using high-viscosity bio-ink. On the other hand, pneumatically driven systems are used regardless of the lightness of the bio-ink due to the possibility of modulating pressure and valve opening time.46, 47
Hydrogels utilized in this type of printing typically belong to the category of non-Newtonian fluids, whose viscosity depends on shear rate and force.48 Typical bio-ink viscosity in this technique can range from 30 MPa/s to even 6×107 MPa/s, with an average resolution of about 100 µm, and some studies indicate that it can reach even 5 µm.23, 32, 49
The main advantage of extrusion-based bio-printing is the ability to print models with very high cell density. Scaffolds obtained in this procedure provide much stronger and more fundamental support in the recovery process after SCI than scaffolds obtained using methods with lower cell density.50 Additionally, this method allows for relatively uniform cell distribution in the print. However, there is a difficulty with the formation of shear stress. Increasing bio-ink concentration and viscosity leads to increased shear stress during extrusion, which leads to reduced cell viability.51 Therefore, optimization of printing parameters is essential for improving cell viability. Recent studies by Smith et al.52 involving co-extrusion of bovine aortic endothelial cells (BAEC) suspended in soluble type I collagen, using a micro-dispersion pen on the hydrophilic side of polyethylene terephthalate sheets, have shown that with proper optimization of conditions, it is possible to obtain a survival rate exceeding 90%.52, 53, 54, 55 Also, this method has been successfully applied to develop tissue engineering constructs, with aortic valves, tumor models and vascular tissues printed in this manner.55, 56, 57, 58
As previously discussed, inkjet printing technology faces a significant challenge in treating SCI due to limitations in printing mode; extrusion printing is the most widely used, enables printing of high-resolution scaffolds and is undoubtedly one of the strongest candidates for treating SCI using bio-printing. On the other hand, light-directed methods (DLP, SLA and LAB) are relatively new. However, due to their ability to produce high-resolution and cell-viable scaffolds with very complex geometric features they represent a potential alternative and hope for the application of bio-printing in spinal cord regeneration.
Injuries, diseases and trauma causing spinal cord deficits
Chronic spinal cord injury
Nerve tissue in the damaged mammalian peripheral nervous system shows the ability to guide axons to synaptic targets based on the removal of myelin debris by immune cells and the secretion of cytokines by Schwann cells. However, SCI leads to scarring composed of myelin, cell debris, microglia, astrocytes, oligodendrocytes, fibroblasts, meninges, and extracellular matrix (ECM) molecules, resulting in impaired axonal regeneration in the damaged area. Scarring is thought to be both a physical and chemical barrier preventing nerve regeneration after SCI.59
Xiao et al.59 in 2016 used intraoperative neurophysiological monitoring to identify and excise scar tissue. The next step was to use a NeuroRegen ‘scaffold’ containing autologous bone marrow mononuclear cells, which were implanted into the resection sites. NeuroRegen bridges the resulting lesion gap and allows the delivery of stem cells or biomolecules to promote neuronal regeneration. In addition to complete loss of motor and sensory function below the injury site, autonomic system dysfunction, including abnormal blood pressure, heart rate control, sweating, and temperature derangement are common clinical consequences of SCI. In the study by Xiao et al., the occurrence of sexual arousal and reduced sweating were observed after application of the NeuroRegen scaffold, indicating partial recovery of autonomic nervous system function. In addition, the return of SSEPs (somatosensory evoked potentials) in the tibia was detected in 2 patients, further evidence of partial nerve regeneration.59
Acute spinal cord injury
The American Spinal Injury Association (ASIA) was established in 1973 to facilitate the exchange of research, data and ideas among practitioners involved in the treatment of patients with SCI (Table 260). Its founders sought to create a standardized model of care for the growing number of patients with SCI.
One of the first applications of scaffolding in SCI is the use of this technique in a 2016 clinical trial. A 25-year-old man sustained a T11–12 fracture after a motocross accident, resulting in a T11 ASIA grade A SCI. He underwent surgical decompression with spinal immobilization and was then included in the study using a bioresorbable scaffold that was implanted into the spinal cord parenchyma directly into the traumatic cavity. After 3 months, the patient’s neurological condition had improved significantly, and the SCI rating had changed from grade A to grade C. Importantly, there were also no surgical complications or apparent safety-related abnormalities following this procedure.61
Another case of successful use of the scaffold is its use in a 2018 clinical trial. Two patients with acute SCI at the T11 and C4 levels, respectively, were assessed as ASIA grade A. After magnetic resonance imaging (MRI) and electrophysiology of the nerves, NeuroRegen collagen scaffolds, which contained human mesenchymal stem cells from the umbilical cord, were implanted at the site of injury. During follow-up, no graft-related adverse events were identified. Return of sensory and motor function was observed in both patients. The level of sensation increased below the vertebrae where the injury occurred and, in addition, the patients regained sensation in the bladder and bowel. The patient with the injury at T11 regained the ability to walk, while the patient with the C4 injury regained the ability to move his toes and lift his legs. In both patients, injury status improved from a complete A grade injury on the ASIA scale to an incomplete C grade.62
Another study (from 2022) used a collagen scaffold transplant that contained the patients’ own bone marrow mononuclear cells or umbilical cord mesenchymal stem cells (UC-MSCs). Fifteen patients with grade A were enrolled in the clinical trial and followed up for 2–5 years. None of the patients experienced serious complications or adverse effects related to the transplantation of the functional scaffold. The study yielded the expected positive results. Five patients with acute SCI achieved an increase in their sensory positions and 6 other patients regained sensation in the bladder. In addition, 4 patients regained their ability to walk.63
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease (NDD) that attacks motor neurons, causing weakness, muscle atrophy and spasticity. The only available treatment options for this condition are only symptomatic. However, an innovative approach using 3D bio-printing and induced pluripotent stem cells (iPSCs) is being investigated. The idea behind the 3D bio-printing solution involves providing the cells with an environment as close to physiological as possible. In a study by Scarian et al.,64 healthy peripheral blood mononuclear cells and amyotrophic lateral sclerosis cells were induced to change into iPSCs and then differentiated into neural stem cells (NSCs) in 2D. In the next step, these cells were printed in 3D hydrogel-based constructs and later induced to differentiate into motor neuron progenitor cells and, in the next phase, into motor neurons. Using confocal microscopy and reverse transcription quantitative polymerase chain reaction (RT-qPCR), cell viability during 3D differentiation was monitored. The results showed no disruption of normal differentiation or electrophysiological features caused by the hydrogel. Characteristic markers at a given stage of differentiation were also investigated, where the difference compared to the 2D environment was the reduced expression of markers such as SOX1, SOX2 and Nestin. Based on this evidence, it was proven that 3D bio-printing can be considered as a good model to study and treat the pathogenesis of amyotrophic lateral sclerosis.64
Summary
To date, treatment of SCI has mainly consisted of surgical bracing of the damaged area, securing it and using drugs to prevent secondary injuries. However, these are not solutions that can permanently restore the functionality of the damaged nerve, and they only reduce the factors causing the injury to a small extent. For this reason, research into 3D bio-printing and scaffolding techniques should be intensively pursued further. Despite their current drawbacks or high costs, in the future, with the advancement of this technique, they may be the main tool capable of fully regenerating a damaged spinal cord.