Abstract
Microencapsulation is a technology for encapsulating particles in a coating designed to isolate the core substance from external conditions, including oxidation, UV radiation or humidity. Microcapsules reach dimensions of up to 5,000 μm. In the pharmaceutical industry, they are used for the controlled release of active substances, masking their taste, odor or gastrointestinal irritation, and can also reduce the toxicity of some medicinal substances. In the food production industry, the encapsulation process applies to sweeteners, enzymes, microorganisms, vitamins and minerals, flavors, or colors. The production of microcapsules is based on the use of their physical properties such as amphiphilicity, partition coefficient and melting point, while their formation of microcapsules is mainly carried out using physical methods such as coacervation, spray drying, cooling and coating, agglomeration, suspension crosslinking, solvent evaporation, and extrusion, as well as chemical methods: interfacial polymerization and in situ polymerization. Although traditional methods are still used to produce microcapsules, contemporary methods employing the latest technology are also emerging. One such method is encapsulation in microcylinders produced with a 3D printer.
Key words: food products, microcapsules, pharmacy, encapsulation methods
Streszczenie
Mikroenkapsulacja jest technologią polegająca na zamykaniu cząstek w powłoce mającej na celu odizolowanie substancji stanowiącej rdzeń od warunków zewnętrznych, m.in. utlenianiem, promieniowaniem UV czy wilgotnością. Mikrokapsułki osiągają wymiary do 5000 mikrometrów. W przemyśle farmaceutycznym są wykorzystywane do kontrolowanego uwalniania substancji aktywnych, maskując ich smak, zapach czy drażniące działanie na układ pokarmowy; mogą również zmniejszyć toksyczność niektórych substancji leczniczych. W przemyśle produktów spożywczych proces enkapsulacji dotyczy substancji słodzących, enzymów, mikroorganizmów, witamin i minerałów, aromatów oraz barwników. Produkcja mikrokapsułek opiera się na wykorzystaniu ich właściwości fizycznych, takich jak amfifilowość, współczynnik podziału czy temperatura topnienia. Tworzenie mikrokapsułek odbywa się głównie za pomocą metod fizycznych, np. koacerwacji, suszenia, chłodzenia oraz powlekania rozpyłowego, aglomeracji, sieciowania w zawiesinie, odparowania rozpuszczalnika oraz ekstruzji, jak również chemicznych: polimeryzacji międzyfazowej oraz in situ.Tradycyjne metody są ciągle wykorzystywane do produkcji mikrokapsułek, wdrażane są też jednak również najnowsze technologie. Jedną z takich metod jest enkapsulacja w mikrocylindrach wytworzonych za pomocą drukarki 3D.
Słowa kluczowe: produkty spożywcze, mikrokapsułki, farmacja, metody enkapsulacji
Introduction
Microencapsulation has its very beginning at the time when the life is born. The emergence of the first cell gives rise to a microcapsule, which is porous in its own specific way. Most eukaryotic cells are excellent examples of capsules that occur naturally in the Universe.1 Natural shells (coatings) formed in this way are extremely effective in fulfilling their roles.2 Among the primary functions of such shells are the protection of the internal material (the core) and the regulation of material flow across the cell membrane. Thanks to such an advanced process, bacterial cells, viruses and even plant spores have managed to survive in extreme conditions for up to 1,000 years.3 An example of this is the black pigment found in fungal cells that protects the core components from sunlight.3 In turn, lipid bilayer systems, being constituents of cell walls, act as valves that allow various types of substances to pass through. The permeability of these membranes for water can be 1,000 times greater than for ions.4 Another natural example of a semipermeable membrane is the chicken egg. The egg shell protectst its contents during incubation, allowing free gas exchange between the developing fetus and the external environment.5 Humans have been trying to replicate mechanisms perfectly functioning in nature for centuries. Researchers have developed microspherical structures capable of selectively isolating their internal environment from the external surroundings. These micro-sized structures, known as microcapsules, exhibit the additional capability of being transported through various biological barriers, such as the skin, bloodstream, or via oral ingestion in the form of food.
The purpose of this review is to present methods for the formation and use of microcapsules in pharmaceutical and food technology as a cross-section through the development of the microencapsulation techniques used starting from the 1930s to the present day.
Encapsulation methods
The first attempts at microencapsulation began in the 1930s at The National Cash Register Company (Dayton, USA).6 One of the problems investigated at that time was how liquids dispersed in solids would behave. The technological development in those years has triggered new challenges, e.g., how to make paper copies without using carbon paper. The development of a paper printing technique free of CO2 emissions (thanks to the use of microcapsules) led to a breakthrough also in research on dispersion and coacervation.7 A concept then emerged to use gelatin as a carrier. In subsequent years, encapsulation processes were improved by implementing new techniques and carriers. As early as in the 1950s, this technique was employed to entrap aromas in order to protect them from oxidation.
Further technological improvement has led to a successful of fortifying orange essential oil in 5-µm coatings, which was undertaken at the Southwest Research Institute (San Antonio, USA) in the 1950s. The resulting product was used in the food industry as a flavor.8 Techniques were further developed that enabled encapsulating the core, i.e., the center of the capsule, with a stable structure in order to protect it from degradation, stabilize it and extend the shelf-life of various substances. Substances encapsulated in this way have been widely used in the food, cosmetic, chemical, paper, pharmaceutical, and agricultural industries.2 On a microscopic scale, microcapsules are the capsules not larger than 5,000 μm,4 whereas the size of nanocapsules should not exceed 100 nm. The sizes of microcapsules have not been explicitly defined in the literature, although some scientific sources indicate that the maximum size of nanocapsules can be up to 5,000 nm due to the formation of agglomerates.
Encapsulation consists in covering materials having micro sizes to protect the encapsulated substance against adverse effects of external factors, like solar radiation, oxygen, humidity, other product components, or effects of the technological process.8 Microencapsulation is a technology of vital importance in the pharmaceutical industry and medicine.9, 10, 11 Microcapsules consist of the core and a coating (shell). The core can be a solid, liquid (solution, suspension or emulsion) or gas. The mass of the core typically makes up 30–95% of the microcapsule’s weight. Microcapsules can be of spherical or irregular shape, depending on core type. Given their structure, they can be divided into monocored, multicored and matrix ones. The monocored microcapsules consist of the core and the coating, whereas the multicored ones are agglomerates of smaller capsules entrapped in one larger capsule. In turn, in the matrix microcapsules, the core is homogenously merged with the coating.12 The encapsulation technique depends on the physicochemical properties of the core substance,13 while the properties of coating components enable a desired profile of a therapeutic substance effect.14, 15 The coating may be composed of synthetic polymers (polyvinylpyrrolidone, polyacrylic acid, polymethacrylate, polyamide) or natural polymers (gelatin, gum arabic, starch, cellulose derivatives, wax, paraffin). Natural biopolymers are one of the most common substances used in encapsulation processes. Their main advantage is the presence of a large number of functional groups, which affects capsule core stabilization, while their other advantages include their low production cost, biodegradability and biocompatibility.16 In addition to its protective effect against external conditions, masking taste and odor, encapsulation also allows for the controlled prolonged release of the drug, thereby reducing its toxicity and alleviating gastrointestinal tract irritation.17 Microcapsules are administered orally14 and externally onto the skin.11, 18
Physical/mechanical methods
Coacervation
Coacervates are nothing more than large micelles that form spontaneously in colloid solutions. First attempts of microencapsulation via phase coacervation were undertaken in 1931 by Bungenburg de Jong and Kass.19 It was thanks to their research that the first gelatin capsules were created. Coacervates are formed by combining oppositely charged particles, such as polysaccharides, proteins or ions. Another definition states that coacervation is the macromolecular separation of a solution into 2 immiscible phases that are in equilibrium. It is important that the ionic substances contained in the mixture differ in their isoelectric points.20 Coacervation can be divided into simple coacervation, the simplified model of which is limited to the use of one dispersed colloidal substance (such as gelatin or chitosan), and complex coacervation, in which an aqueous solution of a polymer and an oppositely charged colloid (e.g., gum arabic) are prepared (Figure 1). It proceeds in 4 stages:
1. dispersion of an active substance in a solution of the active hydrocolloid;
2. addition of the 2nd hydrocolloid in order to induce the polymer–polymer complex (in the case of complex coacervation);
3. colloid precipitation in the form of droplets (by reducing its solubility through external factors, such as temperature or pH changes); and
4. formation and stabilization of capsules by the addition of a crosslinking agent.2
The microcapsules formed are separated from the solution by means of filtration or centrifugation, and then rinsed and dried. The resultant capsules may contain 85–90% of the core21 and be characterized by low porosity and insolubility in cold water. Polymers that can be used in the food industry as core coatings should be suitable for consumption, like, e.g., gelatin, chitosan, shellac, wax, carboxymethylcelluose, gum arabic, or ethylcellulose.22 In the food industry, coacervation can be applied to flavor cakes, teas or oils.20
Spray drying and cooling
The first reports about the use of spray drying for encapsulation appeared in 1927,2 when A. Bolk-Roberts spray dried flavoring oil in acacia gum. Rapid technological progress observed in the 1950s has led to the use of spray drying of milk, coffee, tea, and dyes on a commercial scale.8 Further works on the development of the drying industry have paved the way to the production of stable additives with dextrins dissolved in water used as their carriers. Progress in research has enabled the development of carriers that capture volatile substances, constituting an effective barrier against oxidation and degradation. The drying process consists in dispersing a mixture of a core liquid and a coating substance with a particle diameter of 1–300 µm. The liquid in the drying chamber meets the drying medium (warm air) at a temperature of 100–180°C.23 Despite the high temperatures used, the coating is relatively poorly permeable to volatile compounds. Spray drying occurs in 3 stages:
1. preparation of dispersion or emulsion;
2. homogenization of the material to be dried; and
3. substance spraying in the drying chamber.24
Research works on spray drying have aimed to investigate not only the composition and choice of the carrier but also the influence of various factors on core retention in the capsule, such as the size of the emulsion particles, the type of spraying apparatus, the type of solvent, and the inlet and outlet temperatures.25 Increasing the core concentration can result in greater retention of the component in the capsule. Higher temperature in the chamber also increases its retention but can as well trigger irreversible changes in its chemical structure. The bonds occurring between the core and the coating affect the greater retention of the former.26 The most common substances used to protect the core include gum arabic, maltodextrins, dextrins, modified starches, whey, and rice starch.
Spray freeze drying
Another modern solution in the encapsulation via drying is the spray freeze drying.23 Most often, the mixture to be dried is sprayed in liquid nitrogen, which serves as a freezing medium. Then, the frozen capsules are transferred to the freeze drying chamber, where the solvent evaporates by sublimation. In a study conducted by Sonner et al., this method enabled obtaining single spheres with efficiency similar to that achieved upon traditional spray drying.27 The most common substances used in the spray freezing process as core coating include fats, mixtures of fats, mono- and diglycerides with a melting point of 45–122°C,28 and hardened oils with lower melting temperatures in the range of 32–42°C. Due to their lower melting point, the lipids mentioned above require special storage conditions. Since the coating is hardened by means of a low temperature treatment and not at the temperature of solvent evaporation as in the case of drying, it may be more easily damaged by various mechanical (friction), thermal (temperature increase) as well as chemical (fat reactions) factors.29 The core is released when the temperature is higher than the melting point being its main component. In the food industry, capsules with food additives produced by spray freeze drying are used for instant products, cakes and foods with a higher fat content.30
Fluidized bed drying and coating
Another modification of spray drying process is fluidized-bed drying, in which agglomeration of the core carrier takes place. This method aims to produce capsule agglomerates, which are formed upon dispersed starch binding with a binding agent, e.g., guar gum or pectin.31 The rapid drying of such a mixture results in the formation of agglomerates having pores of various sizes, wherein active substances or drugs can be inserted. Excess substance introduced into the capsule is removed from the surface of the agglomerates using ethanol. The fluidized-bed drying enables the use of other protective coatings of microcapsules. The first mentions of the use of agglomerates in this method date back to the 1950s and were made by D.E. Wurster company; hence, the definition of ‘Wurster coating’ has appeared in the literature.8 Currently, due to high costs, agglomeration and fluidized-bed drying are used in the pharmaceutical and cosmetic industries. Novel technological solutions allow for producing agglomerates of desired sizes by means of higher pressure, suspension flow rate or regulation of nozzle sizes at the chamber outlet.32 Wurster coating is based on an additionally designed chamber with a built-in cylindrical nozzle that sprays the coating material, and the agglomerated particles move upwards in the chamber and pass the nozzle there, where they are encapsulated. The material adheres to the surface of the coating by partial evaporation of the solvent. Excess core is washed out with ethanol immediately after leaving the chamber.
Fluidized-bed coating enables the extension of the controlled release, taste masking, and improvement of stability and aesthetics.33 The main advantages of the agglomeration method include the relatively long stability of the capsules and long release time of the core. The core of capsules produced in the agglomeration process is resistant to oxidation processes, whereas the capsules are highly resistant to external factors. This provides the possibility of encapsulating thermolabile compounds.32 A characteristic feature of agglomerates is their low porosity, which makes them applicable in many industries. However, their major drawback is the high production cost. In addition, due to the composition of agglomerates (mainly starch), the carbohydrate content increases in the final product. In the food industry, this process is used to release substances such as sweeteners, loosening agents, enzymes, microorganisms, vitamins, minerals, flavors, and colorants.34
Suspension crosslinking
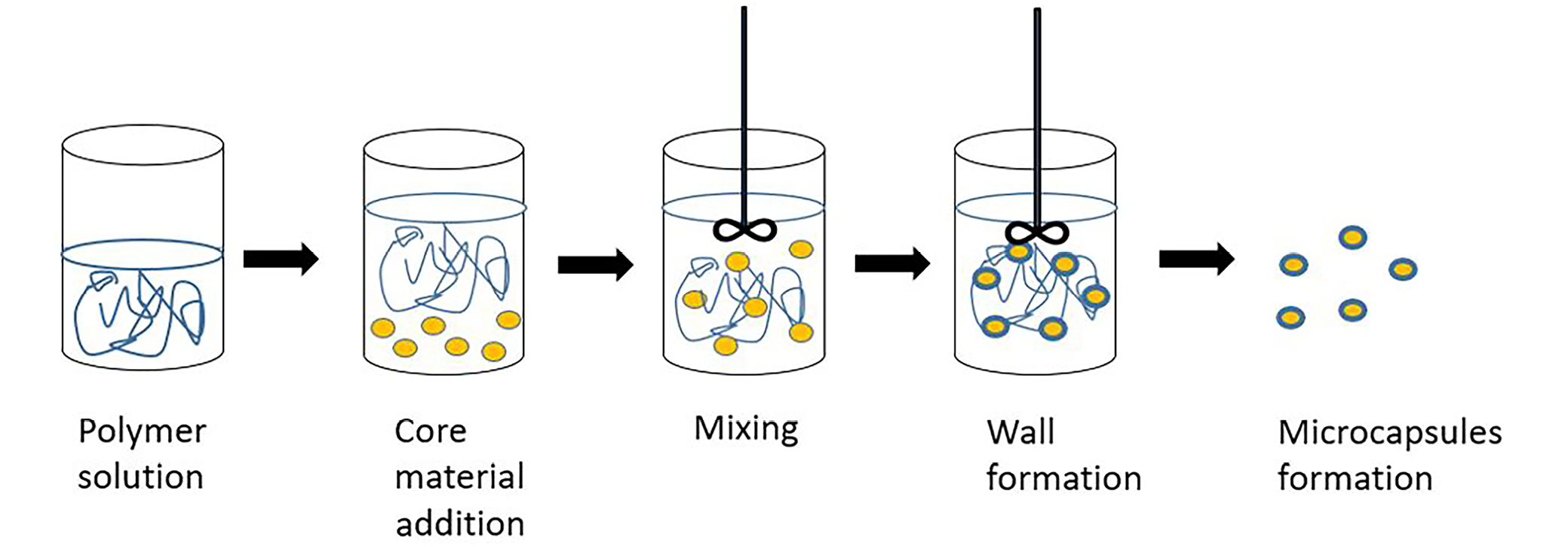
Suspension crosslinking is a preferred method for the preparation of protein and polysaccharide microcapsules.35 It involves dispersing an aqueous solution of the polymer, which contains the core material, in an immiscible organic solvent (suspension/dispersion medium) to form small droplets. The suspension medium typically contains a suitable stabilizer to preserve the integrity of the droplets or microcapsules. The droplets are then hardened with covalent crosslinking, resulting in the formation of the corresponding microcapsules. The crosslinking process can be achieved either thermally (at temperatures above 50°C) or by using a crosslinking agent, such as formaldehyde or terephthaloyl chloride (Figure 2). Suspension crosslinking is a highly versatile technique, suitable for microencapsulation of soluble, insoluble, liquid or solid materials, and can be used to produce both microcapsules and nanocapsules. For instance, albumin nanocapsules containing doxorubicin and magnetite particles have been synthesized using this method.36
Solvent evaporation
Microcapsule formation by solvent evaporation is similar to suspension crosslinking, but typically involves the use of hydrophobic polymers.37 In this method, the polymer is dissolved in a water-immiscible volatile organic solvent (dichloromethane or chloroform), into which the core material is also dissolved or dispersed. The resulting solution is then added dropwise to a stirring aqueous solution containing a suitable stabilizer, such as poly(vinyl alcohol) or polyvinylpyrrolidone, to form small polymer droplets that contain the encapsulated material. Over time, the droplets harden, forming the corresponding polymer microcapsules. The hardening process is achieved by removing the solvent from the polymer droplets through solvent evaporation (using heat or reduced pressure) (Figure 3). The solvent evaporation process is well-suited for the preparation of drug-loaded microcapsules, especially those based on biodegradable polymers. Some of the biodegradable polymers most commonly used as matrices for controlled drug release are poly(lactic acid) (PLA)38, 39 and poly(DL-lactic-co-glycolic acid) (PLGA).40 Microcapsules are produced by dispersing the drug with the polymer and a hydrophilic solvent, such as water or ethanol, using ultrasound. Direct dispersion to form a suspension can occur, as in the case of doxorubicin and PLA.38 The drug can also be pre-dispersed with an emulsifier such as dichloromethane to form a solid-in-oil (s/o) emulsion, in order to be subsequently dispersed with a polymer, as is the case of insulin dispersed using PLGA copolymer.40 In both cases, microcapsules are formed after evaporation of the hydrophilic solvent.
Fortification of substances in the extrusion process
Extrusion is one of the physical methods of encapsulation. It consists in the mechanical extrusion of capsules while mixing the core with the coating agent. Extrusion itself is a mechanical-thermal process, extruding from various bulk materials a plastic mass, which solidifies after some time. This process can be controlled by modifications of temperature, pressure and duration.41 Modifications of other process parameters enable producing a material with specific physicochemical properties. Such parameters are, e.g., the properties of the raw material used, like moisture content, chemical composition, viscosity, or comminution. Further modifications can be applied to the extrusion process parameters, i.e., the speed of screw rotation, temperature, or the number and type of screws.42
The device for microencapsulating substances using the extrusion process differs from typical extruders (Figure 4). It consists of a droplet generator and a tube with a hardening bath.28 Droplets with a core encapsulated in the coating fall into a solution of a hardener or a cross-linking agent (ethyl alcohol, isopropyl alcohol, or ethylenediaminetetraacetic acid). The device contains a narrow capillary through which the core is fed, located in a tube with the coating material. Both materials come into contact with each other at the capillary outlet, disintegrating into individual drops within the liquid. Such device has a high throughput, but the uniformity of the capsules is very low.28
There are also other modifications of this method which allow improving the efficiency and quality of the produced capsules, like, e.g., a spinning head at the capillary outlet placed on the cylinder. In spinning cylindrical extruders, the coating is pumped through a concentric tube, fed by the internal chamber and then injected to the disc circumference.43 Similarly to the previous technological solution, the liquid column disintegrates at the capillary outlet in contact with the coating on the disc periphery. This solution offers several significant benefits, i.e., process temperature control, operation in a controlled atmosphere and no losses.44 It was also noted that the capsule size increases with the increase in the material feeding rate but decreases with the increasing rotational speed of the disc. Both methods can be used to produce capsules that are well soluble in water, with a core content of approx. 10–15%. Their stability can reach up to 5 years, but the simplest solution ensures a stability period of 1–2 years. The encapsulation process by extrusion is carried out at temperatures in the range of 60–120°C.43
The most common capsule coatings are carbohydrates such as maltodextrins, starch and its modified preparations, gum arabic, trehalose, pectin, and sodium alginate.43 Extrusion enables encapsulating not only flavors used in the food industry, but also bacteria, yeasts, proteins, bioactive compounds, or pesticides and enzymes. Extrusion itself is considered a green process, which means that it is an energy-saving, efficient and environmentally friendly solution.45
3D printing
In contrast to the extrusion method for creating microcapsules, scientists are using the latest technologies available. One of these is 3D printing. In the USA, a method for producing microcapsules has been developed that can potentially release drugs for several weeks, called PULSED (Particles Uniformly Liquified and Sealed to Encapsulate Drugs). Using 3D printing, microcylinders made of biodegradable poly(lactic-co-glycolic) acid (PLGA) assembled into arrays are created. In a study by Graf et al., microcapsules filled with labeled dextran were administered to mice in the form of a subcutaneous injection. The compatibility of the PULSED system with proteins and its low cost enabled its multi-faceted applications, from delivering small-molecule drugs to biological therapies and prophylaxis.46
Chemical methods
Polymerization is one of the chemical methods for creating microcapsules. The most popular types are interfacial polymerization and in situ polymerization.
Interfacial polymerization
Interfacial polymerization (formerly referred to as ‘interfacial polycondensation’) was first discovered by Emerson L. Wittbecker and Paul W. Morgan in 1959 as an alternative to the typical high-temperature, low-pressure polymerization technique. This first interfacial polymerization was carried out using the Schotten–Baumann reaction, a method for synthesizing amides from amines and acid chlorides.47 This process involves dissolving reactive monomers or prepolymers in 2 immiscible phases. After the formation of droplets through dispersion, polymerization occurs at the phase boundary, leading to the formation of microcapsules (Figure 5).48 This technique has been employed to produce relatively small capsules (3–6 mm) and has found applications in pharmaceuticals and food products.49, 50, 51 A notable limitation of this approach is that the formation of a thin interfacial polymer layer between the reactants can impede further reaction progress, potentially leading to the formation of microcapsules with compromised mechanical integrity.52 Moreover, the presence of the reactive monomer within the core phase may pose a risk to the encapsulated substances. Additionally, the diffusion of monomers into the core phase may promote the formation of solid microspheres rather than microcapsules.53
In situ polymerization
The in situ polymerization process involves introducing a solution of the monomeric or oligomeric wall material into the core phase, which is then dispersed to the desired size. Polymerization occurs at the phase interface, where controlled deposition and precipitation of the polymer take place. This process can be initiated using precipitants or by altering parameters such as pH, temperature or solvent quality. Depending on the solubility of the monomer and polymer, 3 main types of in situ polymerization are distinguished53:
1. Suspension polymerization occurs when the monomer is insoluble in the dispersion medium, resulting in the formation of suspended monomer droplets that polymerize in the solution to form polymer microparticles. In this case, the reactor conditions and stirring rate are critical for maintaining a uniform particle size distribution (Figure 6);
2. Precipitation polycondensation takes place when the monomer is soluble in the dispersion medium, but the polymer is not. As the reaction progresses, flocculation and aggregation of the polymer (typically of low molecular weight) occur, leading to the formation of particles with a broad size distribution and irregular shape;
3. Dispersion polycondensation is observed when the dispersion medium is a good solvent for the monomer but a poor solvent for the polymer. In this case, polymer swelling occurs, and microcapsule growth takes place through the continuous addition of monomer and oligomer to the particle. This process results in the formation of microparticles with a narrow size distribution.
In situ polymerization can be particularly advantageous when working with volatile54 or less stable substances in emulsion form.55 This technique allows the polymerization to occur directly in the dispersed phase, where the volatile or unstable substances are encapsulated within a polymer matrix. By initiating polymerization in situ, it is possible to stabilize these substances, preventing their degradation or volatilization during processing. Additionally, the controlled polymerization at the phase interface can protect sensitive compounds from environmental factors such as heat, light or oxygen, making it an effective method for handling materials that are difficult to maintain in stable emulsion form. The ability to regulate the in situ polymerization process through variable conditions such as temperature or pH has enabled the use of phase change materials (PCMs) – polymers that alter their conformation in response to changes in physical conditions. Examples of such polymers include poly(N-isopropylacrylamide) (PNIPAM)56 and poly(NIPAM-co-AA).57 These materials can undergo reversible phase transitions, making them suitable for applications where the polymer’s properties need to change in response to environmental stimuli, such as temperature or pH variations.
Conclusions
Microencapsulation has been a technology in use for nearly 100 years for more and more diverse and complex applications, both in the pharmaceutical and food industries. Substances used as carriers of encapsulated substances range from oil to natural and synthetic polymers. Methods for producing microcapsules are also evolving. Initially, scientists used coacervation between particles of colloidal solutions, but now a 3D printer is employed to produce microcylinders. Despite significant developments in microencapsulation technology, traditional methods are still being researched and used in parallel, especially as we return to using natural substances that are biodegradable and easily assimilated by living organisms.












-new.png)
-new.jpg)
-new.png)

-new.png)