Abstract
Globally, skin cancer is the predominant form of cancer, with melanoma identified as its most deadly variant. Projections suggest a surge exceeding 50% in melanoma occurrences by 2040, underscoring the urgency for preventive interventions. Sulforaphane (SFN), a compound found in cruciferous vegetables, is recognized for its cancer-preventive capabilities, particularly against skin cancer. This study employed a rigorous systematic review of various databases, adhering to predefined inclusion criteria for study selection. Data extraction was conducted using a uniform template, and the quality of the included studies was evaluated through the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) risk of bias tool, specifically designed for animal research. The review encompasses studies published in English from 2000 to 2023, culminating in the inclusion of 9 pertinent studies. The findings highlight SFN’s capacity to act as a protective agent in preventing skin cancer in animal models. It demonstrated efficacy in curbing skin tumorigenesis triggered by assorted carcinogens, reducing the onset of skin tumors and impeding the growth and spread of skin cancer cells. Furthermore, SFN showed preventive effects against UVB-induced skin carcinogenesis by obstructing the activator protein 1 signaling pathway. Based on evidence from animal-based research, SFN emerges as a promising chemopreventive substance against skin cancer. Nevertheless, determining its optimal dosage, application duration and method of administration for human subjects remains pending. If its effectiveness is substantiated, SFN could complement or offer an alternative to existing preventive measures against skin cancer.
Key words: sulforaphane, skin cancer, animal models, chemoprevention
Introduction
In 2020, the global incidence of melanoma was estimated at approx. 325,000 cases, resulting in about 57,000 fatalities. The International Agency for Research on Cancer (IARC) forecasts a significant surge in the incidence of cutaneous melanoma by over 50%, reaching more than 500,000 annual cases by 2040, with fatalities anticipated to increase by over 2/3 to nearly 100,000 per annum.1 Despite the preventable nature of many instances, cutaneous melanoma represents the most lethal form of skin cancer, comprising approx. 20% of all skin cancer diagnoses. Skin cancer is the most frequently diagnosed cancer globally, with an estimated 1.5 million new cases reported in 2020.1
The predominant forms of skin cancer include basal cell carcinoma, squamous cell carcinoma and melanoma.2 A primary risk factor for skin cancer development is ultraviolet (UV) radiation exposure from the sun, with the risk increasing cumulatively over time.2 While most skin cancer cases are treatable through surgical or alternative therapeutic interventions, prevention plays a pivotal role in reducing the disease’s burden.3 Among the preventative strategies, the use of sunscreen and protective clothing has proven effective in reducing the risk of skin cancer onset. Nevertheless, there is an urgent need for additional preventative measures, particularly for individuals at high risk of the disease.
A variety of promising phytochemicals, such as epigallocatechin-3-gallate, resveratrol, curcumin, pro-anthocyanidins, silymarin, apigenin, capsaicin, genistein, indole-3-carbinol, and luteolin, derived from various fresh fruits, vegetables, roots, and herbs, have been identified to enhance cancer chemoprevention and treatment through diverse mechanisms.4 Sulforaphane (SFN), an isothiocyanate naturally occurring in cruciferous vegetables such as broccoli, Brussels sprouts and cabbage, has demonstrated chemopreventive properties against various cancers, including skin cancer.5 Sulforaphane is known for activating the nuclear factor erythroid 2–related factor 2 (Nrf2) pathway, implicated in cellular defense against oxidative stress and inflammation.6 Activation of the Nrf2 pathway facilitates the induction of phase 2 detoxifying enzymes, aiding in the prevention of carcinogen formation and promoting their elimination from the organism.7
Preclinical investigations have explored SFN’s efficacy against skin cancer in animal models. For instance, SFN has been shown to inhibit skin tumor growth in mice exposed to the carcinogen 7,12-dimethylbenz(a)anthracene (DMBA).8 Furthermore, it has provided protection against UV radiation-induced skin carcinogenesis in SKH-1 high-risk mice9 and has been shown to prevent the development of skin tumors in mice by inhibiting the promotion stage of skin carcinogenesis.10
Topical photodynamic therapy (PDT) with 5-aminolevulinic acid (ALA) is commonly used to treat non-melanoma skin cancers, actinic keratoses and various dermatoses. However, it may cause adverse effects, such as pruritus, erythema, edema, and pain. The compound (R)-L-SFN has been found to reduce erythema while inducing DNA fragmentation, leading to apoptotic cell death.11 Another investigation assessed SFN’s impact on protoporphyrin IX (PpIX) production and PDT efficacy, revealing that SFN did not affect PpIX photodegradation and increased PpIX synthesis in human skin, although not in A431 cells. The findings suggest that (R)-L-SFN pre-treatment prior to topical ALA-PDT could enhance ALA penetration through the stratum corneum, thereby increasing PpIX synthesis.11 Although preclinical studies have shown promising results, more research is needed to understand the potential benefits of SFN for the prevention and treatment of skin cancer in humans. This systematic review aims to collate and analyze the existing evidence regarding the application of SFN in skin cancer across preclinical and clinical studies.
Methodology
This study was meticulously designed following the PROSPERO guidelines, which establish the gold standards for conducting systematic reviews. Registration with PROSPERO, under the No. CRD42023417867, ensured transparency and compliance with the established protocol. The primary aim of this systematic review was to examine the existing scientific literature on the effectiveness of SFN in preventing and treating skin cancer in animal models. To conduct a comprehensive literature survey, searches were conducted across multiple databases, including PubMed, Science Direct, Embase, and Google Scholar. The search strings included combinations of keywords such as “sulforaphane”, “skin cancer”, “nonmelanoma skin cancer”, “squamous cell carcinoma”, “basal cell carcinoma”, “melanoma”, “animal models”, and “preclinical studies”. Boolean operators (AND, OR) were used to refine the search results. For instance, the PubMed search string was “sulforaphane AND (skin cancer OR melanoma OR squamous cell carcinoma OR basal cell carcinoma) AND (animal model OR preclinical study)”. Discrepancies in study inclusion were resolved through discussions between the 2 authors. The search parameters were limited to studies published in English from 2000 to 2023, initiating the search in April 2022 and updating it in March 2023.
The inclusion criteria for this review were rigorously defined. We primarily selected studies that examined the impact of SFN on skin cancer using animal models. Studies were required to provide detailed descriptions of the methodologies used, and they needed to include at least 1 measure evaluating the chemopreventive effects of SFN. Only peer-reviewed scientific journal publications were included in our analysis.
A standardized form was utilized for data extraction from the selected studies, capturing vital information such as study design, animal models used, sample sizes, intervention types, assessed outcomes, and resulting conclusions.
Risk of bias assessment
In this systematic review, the internal validity of preclinical animal studies was evaluated using the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) risk of bias tool, as outlined by Hooijmans et al. in 2014.12 This comprehensive tool scrutinizes 10 critical domains to assess the risk of bias, namely sequence generation, baseline characteristics, allocation concealment, random housing, blinding, random outcome assessment, incomplete outcome data, selective outcome reporting, other sources of bias, and overall risk of bias.
The assessment revealed a spectrum of bias risks across the included studies. For instance, the studies by Abel et al. and Shibata et al.13, 14 were characterized by low-risk ratings in all domains, indicating methodological rigor and reliability. In contrast, studies by Alyoussef and Taha,15 Dinkova-Kostova et al.9 and Gills et al.10 exhibited higher or indeterminate risks of bias in certain areas. Notably, blinding emerged as a recurring concern, with many studies receiving high or ambiguous ratings for this domain, suggesting potential vulnerabilities in their designs. The bias assessment revealed variability in methodological quality across studies, particularly in sequence generation and allocation concealment, indicating a need for improved randomization processes. Additionally, blinding of outcome assessment was another area with a high risk of bias, particularly in studies by Alyoussef and Taha15 and Gills et al.10 These biases could potentially influence the observed effects of SFN on skin cancer. Detailed findings are summarized in Table 1,8, 9, 10, 13, 14, 15, 16, 17, 18 with annotations on studies exhibiting high or unclear risk in specific domains. Although the SYRCLE risk of bias tool provides a structured approach for identifying potential biases in preclinical animal studies, it is essential to acknowledge that no evaluative mechanism can entirely eliminate bias or confounding variables. Nonetheless, the application of the SYRCLE risk of bias tool is instrumental in identifying and, where possible, mitigating biases, thereby enhancing the internal validity of the preclinical animal research under review.
Results
Search results
The initial search across 4 databases (PubMed, Science Direct, Embase, and Google Scholar) for studies concerning SFN and skin cancer in animals produced a total of 5,661 records. Among these, 143 duplicate records were removed, and 4,222 records were marked as ineligible by automation tools. Additionally, 894 records were excluded for various reasons, resulting in 402 records remaining for screening. During the screening process, 386 records were excluded for reasons such as failing to meet inclusion/exclusion criteria or being associated with cell line studies or human clinical trials. After the eligibility assessment, 16 reports were identified for retrieval, all of which were retrieved. These 16 reports were then screened in their entirety, leading to the exclusion of 7 reports. Exclusion criteria included 2 reports not meeting the inclusion/exclusion criteria, 3 being related to cell line studies, and 1 being a human clinical trial. Subsequently, a Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) chart was created to reflect these findings (Figure 1). Summary of preclinical studies on the effects of SFN in various animal models of skin tumorigenesis is presented in Table 2.8, 9, 10, 13, 14, 15, 16, 17, 18
Sulforaphane in skin cancer: Molecular pathways and mechanisms
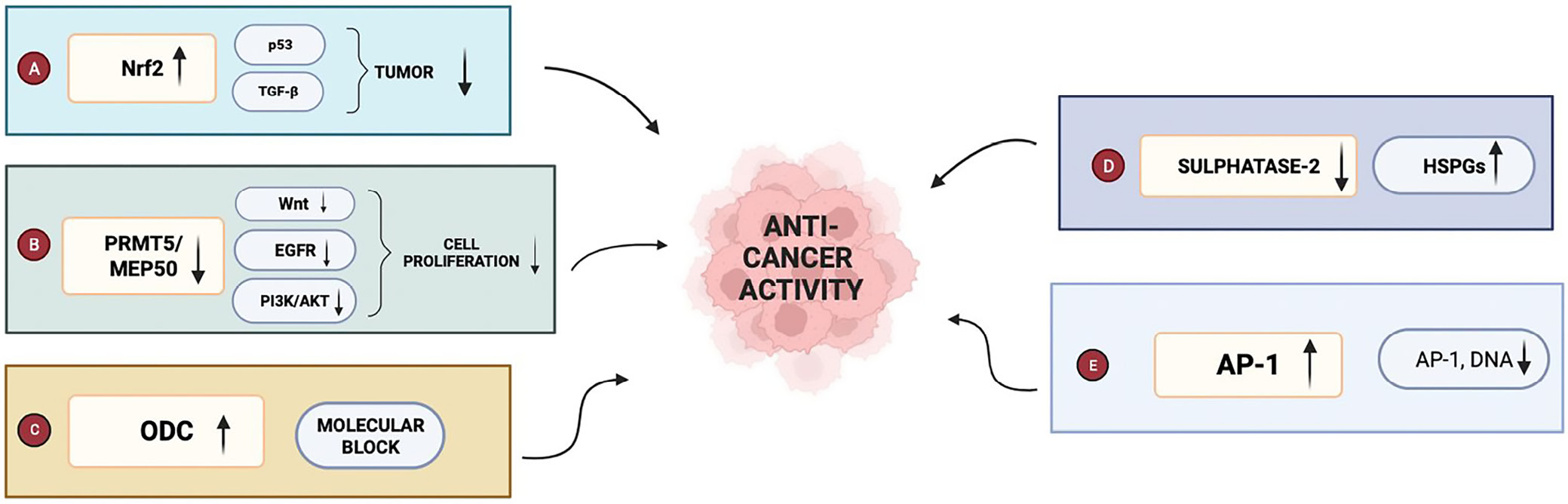
In the context of skin cancer, SFN exerts its potent anticancer effects by intricately modulating various molecular pathways and targets. These include the Nrf2 pathway, where SFN acts as a robust activator, facilitating the nuclear translocation of Nrf2 to interact with antioxidant response elements in the DNA. This instigates the transcription of genes encoding antioxidant and detoxification enzymes, thereby shielding cells from oxidative insults. Sulforaphane also activates the p53 pathway, a pivotal tumor suppressor pathway orchestrating cell growth and division through mechanisms involving apoptosis induction, cell cycle arrest and DNA repair. Additionally, SFN exerts an inhibitory influence on the Wnt pathway, responsible for cellular proliferation, differentiation and migration, by suppressing key Wnt signaling proteins. Sulforaphane also intervenes in the transforming growth factor beta (TGF-β) pathway, impeding the activation of TGF-β signaling receptors, thereby affecting various cellular processes, including growth, differentiation and apoptosis. Moreover, SFN regulates the EGFR pathway, which governs cell proliferation, differentiation and survival, by downregulating EGFR and its downstream signaling molecules. Lastly, SFN plays a pivotal role in modulating the PI3K/AKT pathway, associated with cell proliferation, survival and growth, by blocking the activation of PI3K and AKT. These actions collectively underscore the multifaceted and promising potential of SFN as an agent for skin cancer prevention and therapy. The above mechanisms are shown in Figure 2.
Discussion
This systematic review aimed to assess the effectiveness of SFN in mitigating skin cancer in animal models. It analyzed 10 research studies that explored the impact of SFN on various aspects of skin cancer, including tumor initiation, growth and the underlying molecular processes contributing to cancer advancement. The evidence from these studies collectively indicates a potential protective role of SFN against skin cancer in animal models. Notably, SFN administration was found to mitigate skin tumorigenesis in mouse models exposed to carcinogens such as 7,12-dimethylbenz(a)anthracene (DMBA) and ultraviolet B (UVB) radiation. Xu et al.8 elucidated that SFN suppresses DMBA-induced skin tumors in C57BL/6 mice by activating Nrf2. Recent research has highlighted Nrf2’s role in modulating antioxidant, detoxifying and drug-metabolizing enzymes, thereby conferring SFN’s chemopreventive potential.19 Similarly, Dinkova-Kostova et al.9 demonstrated that SFN-enriched broccoli sprout extracts confer protection against UVB-induced skin carcinogenesis in SKH-1 high-risk mice by inducing phase-2 detoxifying enzymes.
The inhibitory effects of SFN on the proliferation and invasion of epidermal squamous cell carcinoma (SCC) cells further underscore its chemopreventive efficacy. Saha et al.16 reported that SFN curtails SCC tumor formation by downregulating protein arginine methyltransferase 5 and methylosome protein 50, proteins implicated in cancer prognosis and epigenetic regulation.20 Additionally, SFN’s blockade of sulfatase-2, an enzyme with oncogenic properties in human cell lines,21 significantly reduced melanoma cell growth and metastasis in mouse models.15 Moreover, SFN exhibits an ability to prevent skin tumorigenesis during the critical tumor promotion stage. Dickinson et al.17 found that SFN treatment attenuates the expression of proinflammatory cytokines, including interleukin (IL)-1β, IL-6 and tumor necrosis factor alpha (TNF-α), thereby inhibiting tumor promotion. This anti-inflammatory action is complemented by SFN’s inhibition of the activator protein 1 pathway, a mechanism proposed to underlie its protective effect against UVB-induced skin cancer.22
Emerging clinical evidence suggests the therapeutic potential of SFN in the treatment of skin cancer. A clinical trial conducted by Tahata et al.18 assessed the safety and efficacy of broccoli sprout extract containing SFN in patients with atypical nevi and a history of melanoma. The study reported dose-dependent increases in SFN levels in plasma and skin, accompanied by reductions in proinflammatory cytokines and an increase in tumor suppression, advocating for further investigation into SFN as a chemopreventive agent for melanoma.
The collective findings from preclinical and preliminary clinical studies underscore SFN’s potential as a chemopreventive agent against skin cancer, mediated through multiple mechanisms, including the modulation of carcinogen metabolism, inhibition of cell proliferation and inflammation, and the blockade of oncogenic pathways. Further research, particularly clinical trials, is warranted to fully elucidate SFN’s therapeutic efficacy and mechanism of action in skin cancer prevention and treatment.
Limitations
This systematic review was subject to several limitations. First, the inclusion criteria, which only allowed English-language studies, may have introduced language bias. Second, the variability in animal models and SFN dosages across studies complicated direct comparisons and a meta-analysis. Additionally, the predominance of preclinical studies necessitates cautious interpretation when extrapolating to human contexts.
Conclusions
The reviewed studies indicate SFN’s potential for skin cancer prevention, but further research is needed to ascertain its optimal dose, duration and administration method. Clinical trials are essential to assess its effectiveness and safety. If successful, SFN could complement existing prevention measures such as skin checks and sunscreen use.