Abstract
Background. Today’s growing demand for advanced and sustainable polyester materials is driven by an increasing awareness of the environmental impact of traditional materials, emphasizing the need for eco-friendly alternatives. Sustainability has become central in materials development, including the biomedical area, where biobased and environmentally friendly solutions are a rapidly growing field.
Objectives. This research aims to comprehensively evaluate a new enzymatically catalyzed furan-based copolymer, poly(decamethylene furanoate)-co-(dilinoleic furanoate) (PDF-DLF), with a 70–30 wt% hard-to-soft segment ratio. Then, its performance across medical applications is explored, with a particular focus on its potential as a nanofibrous scaffolding material.
Materials and methods. PDF-DLF was synthesized from biobased monomers using Candida antarctica lipase B (CAL-B) as the biocatalyst. Material characterization included dynamic mechan-ical thermal analysis (DMTA) to assess the mechanical behavior and thermal properties. Enzymatic degradation studies determined biodegradability, while cytotoxicity tests established in vitro biocompatibility. The copolymer was electrospun into nanofibers, with scanning electron microscopy (SEM) employed to analyze their morphology.
Results. PDF-DLF displays mechanical and thermal properties indicating high storage modulus and 2 main temperature transitions. Enzymatic degradation studies and cytotoxicity assessments confirm biodegradability and in vitro biocompatibility. Electrospinning successfully transformed the copolymer into nanofibers with diameters ranging from 500 nm to 700 nm.
Conclusions. This study significantly advances our understanding of sustainable polyesters with versatile processing capabilities. The successful electrospinning highlights its potential as a biodegradable scaffold for medical engineering, supported by biocompatibility and sufficient mechanical properties. It opens new opportunities for sustainable materials in critical biomedical industries, including tissue engineering.
Key words: 2,5-furandicarboxylate, enzymatic synthesis, bio-based monomers, CAL-B, block copolymers
Streszczenie
Wprowadzenie. Współcześnie rośnie zapotrzebowanie na zaawansowane materiały poliestrowe, które nie tylko wykazują nowatorskie właściwości funkcjonalne, ale także spełniają podwyższone standardy zrównoważonego rozwoju. Wymagania te stają się kluczowe dla rozwoju materiałów, z inżynierią biomateriałów włącznie, gdzie rozwiązania oparte na naturalnych i zrównoważonych surowcach stają się dynamicznie rozwijanym obszarem.
Cel pracy. Celem badań jest ocena i charakterystyka katalizowanego enzymatycznie kopolimeru – poli(furanianu dekametylenu)-co-(furanianu dilinolu) (PDF-DLF) o stosunku segmentów sztywnych do giętkich 70–30 wag% oraz zbadanie jego potencjału w zastosowaniach biomedycznych jako nanowłókniste skafoldy.
Materiał i metody. PDF-DLF został zsyntetyzowany z monomerów pochodzących z biomasy na drodze dwuetapowej syntezy w eterze difenylu, wykorzystując lipazę B Candida antarctica jako biokatalizator. Termiczna analiza dynamicznych właściwości mechanicznych (DMTA) pozwoliła na ocenę właściwości mechanicznych i termicznych. Badania degradacji enzymatycznej określiły podatność materiału na biodegradację, a ocena cytotoksyczności in vitro umożliwiła ocenę biokompatybilności. Kopolimer został poddany procesowi elektroprzędzenia w celu wytworzenia nanowłókien, których morfologię oceniono za pomocą skaningowego mikroskopu elektronowego (SEM).
Wyniki. PDF-DLF wykazuje interesujące właściwości mechaniczne i termiczne, wskazujące na wysoki moduł zachowawczy i dwie przemiany temperaturowe. Badania degradacji enzymatycznej i ocena cytotoksyczności potwierdziły biodegradowalność i biokompatybilność materiału. Stosując metodę elektroprzędzenia wytworzono nanowłókna o średnicy mieszczącej się w zakresie od 500 do 700 nm.
Wnioski. Przeprowadzone badania wskazują na szerokie możliwości przetwórcze nowego kopolimeru. Otrzymany materiał jest biodegradowalny, biokompatybilny oraz posiada odpowiednie właściwości mechaniczne oraz termiczne. Co więcej, możliwość przekształcenia materiału w nanowłókna podkreśla jego potencjał zastosowania w inżynierii biomedycznej jako rusztowania dla inżynierii tkankowej.
Słowa kluczowe: kopolimery blokowe, synteza enzymatyczna, bio-pochodne monomery, CAL-B, kwas furanodiakarboksylowy
Background
In the ever-evolving field of biomedical engineering, the quest for sustainable materials that are both environmentally friendly and functionally versatile has never been more necessary. Traditional polymers often rely on petroleum-derived compounds, which not only contribute to environmental pollution but also raise concerns regarding their biocompatibility in biomedical applications.1, 2
In this context, 2,5-furandicarboxylic acid (FDCA) emerges as a promising candidate with its unique potential derived from natural resources, offering an alternative to petrochemical monomers.3, 4 The FDCA is a carbohydrate compound within the furfural group, derived from polysaccharides, starch or lignocellulose. Its chemical structure bears resemblance to terephthalic acid (TPA), the primary petrochemical monomer for the synthesis of semicrystalline aromatic polyesters like poly(ethylene terephthalate) (PET) or poly(butylene terephthalate) (PBT).5, 6, 7, 8 Notably, numerous publications have demonstrated FDCA’s capacity to yield materials with superior properties and enhanced polymer chain orientation compared to their TPA analogs, which imparts significant advantages.9, 10, 11, 12 Such attributes are particularly critical in the field of tissue engineering, where the mechanical properties of the material must often align with the specific tissue being repaired or replaced.
Interestingly, the versatility of copolymers containing both hard and soft segments offers the advantage of tailoring the final properties to meet the precise requirements of different tissue engineering applications.13
A growing interest in the conversion and utilization of biomass, coupled with rapid developments in the biorefinery industry, has introduced a wide spectrum of opportunities in the production of novel monomers and polymers from renewable sources.14, 15, 16, 17, 18, 19 However, questions persist about the selection of the best and most selective catalyst. Conventionally, PET material is produced in melt processes using antimony-based catalysts in the form of oxide or acetate, known for their high performance and ability to withstand elevated temperatures. Nevertheless, a significant drawback arises from the presence of residual metals within the polymeric material, which is challenging to remove due to strong metal–ester interactions. This can lead to material discoloration and raise environmental concerns upon disposal, potentially disqualifying the polymer’s use in various applications, including the medical industry.16, 20
To address this issue, we used enzymes that have emerged as a compelling alternative catalyst derived from natural resources. Among these enzymes, Candida antarctica lipase B (CAL-B) stands out due to its stability and high reactivity, with its unique structure enabling high region-, enantio- and stereoselectivity, and the ability to function under mild reaction conditions.21, 22, 23, 24 As confirmed in previous studies, enzymatic catalysis results in a more regular structure in copolyesters compared to metal-catalyzed processes.25, 26 This feature opens up opportunities to obtain optically pure materials with well-defined chemical structures, particularly valuable in the pharmaceutical industry. The CAL-B-induced polymerization has already been successfully applied to various polymer types, including polyesters based on 2,5-bis(hydroxymethyl)furan, vegetable oils, sugars, and 3,6-dianhydrohexitol.17, 27, 28, 29, 30 By using monomers and catalysts originating from biomass resources, it is possible to provide sustainable and safe materials for biomedical applications.
Advances in the tissue engineering field have brought much attention regarding scaffold fabrication, such as with biodegradable polyester nanofibers. The justification for using nanofibers for tissue engineering is that the nonwoven polymeric meshwork is a close representation of the nanoscale protein fiber meshwork in the native extracellular matrix (ECM).31 The electrospinning technique is a promising way to fabricate a controllable, continuous nanofiber scaffold mimicking the ECM structure. Electrospun nanofibers provide a high surface-to-volume ratio and high porosity as a promising scaffold for tissue engineering. Because the degradation behaviors of scaffolds significantly affect new tissue regeneration, the degradation of the material becomes one of the crucial factors when considering using polyester nanofibers as tissue engineering scaffolds.
Objectives
Therefore, we focused on a comprehensive exploration of furan-based copolymers, investigating their potential as scaffolds in the dynamic field of biomedical engineering, and emphasizing their ecological advantages and the integration of enzymatic catalysis for improved sustainability. Our focus extends to the electrospinning technique applied to poly(decamethylene furanoate-dilinoleic furanoate) (PDF-DLF) with 70 wt% PDF segments, introducing a groundbreaking avenue for the creation of sustainable biomaterials. This innovative approach aims to not only assess the biocompatibility and biodegradation profile of the manufactured material but also to evaluate its suitability for processing into nanofibers.
Materials and methods
Materials
The following chemicals were purchased from Sigma-Aldrich (Poznań, Poland): diphenyl ether (DE; ≥99%), mouse fibroblasts L929 EACC, Dulbecco’s modified Eagle’s medium (DMEM), Dulbecco’s phosphate-buffered saline (DPBS), resazurin, penicillin, streptomycin, fetal bovine serum (FBS), and L-glutamine. Polycaprolactone CAPA 6430 was purchased from Perstop (Warrington, UK). 1,10-decanediol (DDO; ≥99%) was acquired from Acros Organics (Geel, Belgium). Dimethyl 2,5-furandicarboxylate (DMFDCA; ≥99%) was obtained from Fluorochem (Łódź, Poland). Dilinoleic diol (DLD; ≥96.5%) (trade name Pripol™ 2033) was obtained from Cargill Bioindustrials (Gouda, the Netherlands). Chloroform (≥98.5%) was purchased from Chempur (Piekary Śląskie, Poland), and methanol (≥99.8%) was acquired from Stanlab (Lublin, Poland). CAL-B covalently immobilized on polyacrylate beads (300–500 µm, ≥95%; Fermase CALB™ 10000), with a nominal activity of 10 000 PLU/g (propyl laurate units per gram dry weight) was purchased from Fermenta Biotech Ltd (Mumbai, India) and Enzyme Catalyzed Polymers LLC (Akron, USA). Before use, CAL-B was pre-dried under vacuum for 24 h at 40°C, and diphenyl ether was stored over 4Å molecular sieves. The remaining chemicals were used as received.
CAL-B catalyzed polycondensation
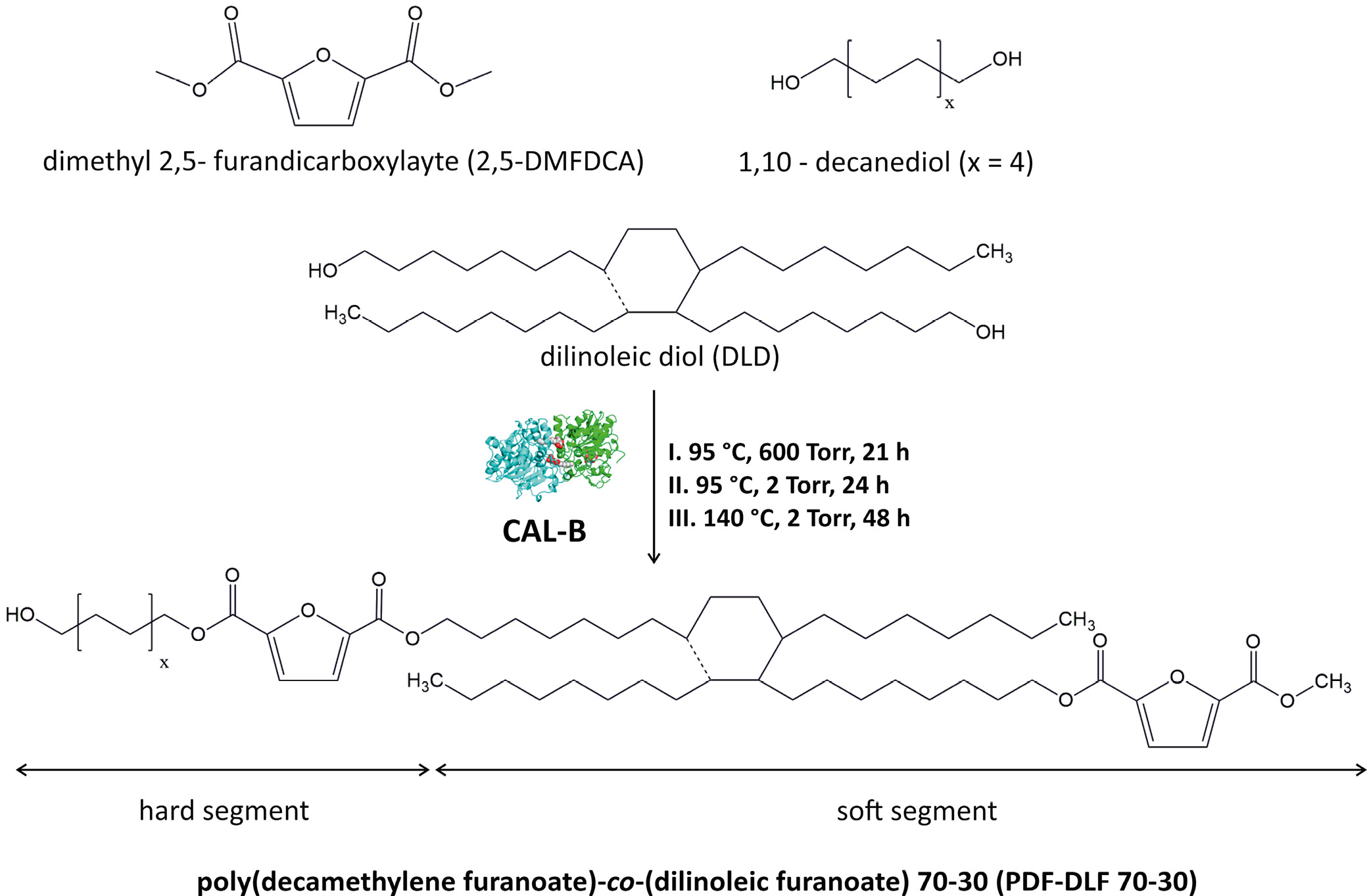
To produce poly(decamethylene furanoate)-co-(dilinoleic furanoate) (PDF-DLF) copolyester with a 70–30 wt% hard to soft segment ratio, a 2-step polycondensation reaction was carried out in diphenyl ether at different temperatures, following the protocol outlined in our prior study.32
Dynamic mechanical thermal analysis
To determine the storage and loss modulus, a PDF-DLF sample for dynamic mechanical thermal analysis (DMTA) was fabricated by melt-pressing within a temperature of 110°C. The specimen had dimensions of 100 µm in thickness, 1 mm in width and 50 mm in length. The measurements were carried out in tensile mode using the DMA Q800 apparatus (TA Instruments, New Castle, USA). The analysis was conducted at a constant frequency of 1 Hz, a heating rate of 2°C/min and an amplitude of 60.
Enzymatic degradation
Enzymatic degradation studies were conducted on melt-pressed films (prepared according to the same protocol as for DMTA studies) using lipase produced by Pseudomonas cepacia bacteria. The enzyme was prepared in DPBS sterile buffer with a concentration of 25 U/mL and a pH of 7.25. Sodium azide (0.02%) was added to prevent the bacteria from multiplying. Degradation was carried out at a temperature of 37°C for 35 days, and measuring points were scheduled every 7 days. The degradation medium was replaced every 48 h to maintain enzyme activity. Each measuring point consisted of 4 samples, which were subjected to weight, microscopic and size exclusion chromatography (SEC) analysis. Enzymatic degradation was performed on discs of the test material (6 mm in diameter and 100 µm in thickness). To ensure sterile conditions during the enzymatic degradation tests, all operations were performed under a laminar airflow chamber. The discs of both the test and reference materials were sterilized with UVC radiation for 30 min on each side.
To monitor the enzymatic degradation progress, the samples underwent analysis for mass loss, molecular weight, morphology, and structural changes. Mass loss was quantified using Equation (1), where m0 is the sample mass before degradation and md is the mass of the vacuum-dried sample (37°C, −60 kPa, 48 h) after degradation. Molecular weight analysis was performed via SEC using a Thermo Fisher (HLC-8320) system (Thermo Fisher Scientific, Waltham, USA) equipped with 4 detection systems: a dual absorbance UV (UV; Waters 2487; Waters Corporation, Santa Barbara, USA; wavelength λ = 254–280 nm), multi-angle laser light scattering (MALLS, Wyatt DAWN; Waters Corporation), a refractive index detector (RI; Wyatt Optilab®; Waters Corporation), and a viscometer (VIS; Wyatt ViscoStar®; Waters Corporation) detectors. The determination of average molar (apparent) mass (Mn, Mw) and dispersity (Đ, Mw/Mn) was carried out through gel chromatography. The mobile phase used was CHCl3 (HPLC grade; Alfa Aesar, Haverhill, USA) at a flow rate of 1 mL/min, a sample concentration of 15 mg/mL and an injected volume of 50 μL. Before injection onto the column, the sample solutions underwent filtering using a 0.20 µm sulphate removal plant (SRP; to extract the sulphates from prior to injection) 20 filter. Absolute molecular masses were derived by processing the data with ASTRA v. 8.1.2 software (Waters Corporation), assuming 100% recovery from the columns.
Structural alterations were evaluated through attenuated total reflection–Fourier-transform infrared (ATR–FTIR) spectra, recorded on a Bruker ALPHA spectrometer (Bruker Corporation, Billerica, USA) within the spectral range of 400–4000 cm−1, employing a resolution of 2 cm−1. Notably, 32 scans were conducted for each sample. Morphological changes were examined utilizing a scanning electron microscope (SEM) equipped with ultra-high resolution (UHR; Hitachi SU8020; Hitachi Ltd., Chiyoda, Tokyo, Japan).
Mass loss = (m0 – md)/m0 × 100% (1)
Cytotoxicity assessment
The cytotoxic potential and growth-inhibitory effects of the PDF-DLF copolyester were assessed in cell culture using L929 mouse fibroblasts, following ISO 1993-5 guidelines. L929 cells (passages 9–11) were cultured in growth media consisting of DMEM, 10% FBS, 2 mM L-glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin in T25 flasks. For the experiments, sub-confluent T25 culture flasks of L929 cells were trypsinized, and 1×104 cells were seeded per well in a 96-well plate. Simultaneously, 100-µm-thick films of PBF-DLF copolyester and a reference material (polycaprolactone PCL CAPA® 6430; Pertstrop, Warrington, UK) were cut into three 6 cm2 samples, which were then sterilized under UV-C light for 15 min on each side. Subsequently, the material samples (n = 3) were sectioned into smaller pieces and placed in a 24-well plate, with 1 mL of medium added to each well. The plates were incubated for 24 h at 37°C in a 5% CO2 environment to allow the cells to adhere and spread. After incubation, the media was aspirated, and 100 µL of growth media containing extracts from the tested materials were added to the wells (with 6 technical replicates performed for each material). A sham control was prepared by adding 100 µL of pure growth media. The plate was incubated for an additional 24 h, after which cell viability was evaluated using an inverted light microscope (Delta Optical IB-100; Delta Optical, Mińsk Mazowiecki, Poland) and a resazurin viability assay.23 The assay was conducted with a fluorescent plate reader (Biotek Synergy HTX; BioTek, Winooski, USA) at an excitation wavelength of 540 nm and an emission wavelength of 590 nm. During the resazurin viability assay, complete growth media was added to an empty well without cells to serve as the blank. The results obtained were expressed as the percentage of normalized cell viability (CV%), calculated using Equation (2)33:
CV% = (FLs – FLb)/(FLc – FLb) × 100% (2)
where FL is the fluorescence intensity and indices s, b and c refer to sample, blank and control, respectively.
Electrospinning
A systematic exploration of key parameters was conducted to optimize the electrospinning process for the synthesis of our novel polymer. The homemade electrospinning setup employed in this study comprised a syringe pump, a high-voltage power supply and a collector. Various parameters, namely needle diameter, applied voltage, flow rate, distance between the needle tip and the collector, and polymer solution concentration, were subjected to meticulous optimization. The distance between the needle tip and the collector varied at 10 cm, 15 cm and 20 cm, while the applied voltage ranged from 10 kV to 20 kV. Simultaneously, the flow rate of the polymer solution was adjusted at 2 mL/h, 5 mL/h and 10 mL/h. A high-purity solvent, chloroform, was utilized for the polymer solution, and concentrations spanning from 1% to 50% were explored, with observations facilitated using a light microscope Delta Optical Evolution 300 (Delta Optical). The morphology of resulting fibers was examined using a SEM on a Hitachi SU8020 (Hitachi Ltd.) apparatus with UHR.
Results and discussion
Fully biobased PDF-DLF copolymer containing 70 wt% PDF as the hard segments and 30 wt% DLF as the soft segments were successfully synthesized employing temperature-varied 2-step method in diphenyl ether using CAL-B as biocatalyst according to the protocol described in our previous paper32 and as presented in Figure 1. In the aforementioned study, we also conducted a comprehensive assessment of the chemical structure, molecular weight, as well as thermal and crystalline properties. A summary of the essential material parameters is given in Table 1. Within the context of this research, we continued our investigation of the PDF-DLF material, with a particular emphasis on evaluating additional critical characteristics that are essential in determining the feasibility of utilizing furan-based copolymers in the biomedical sector.
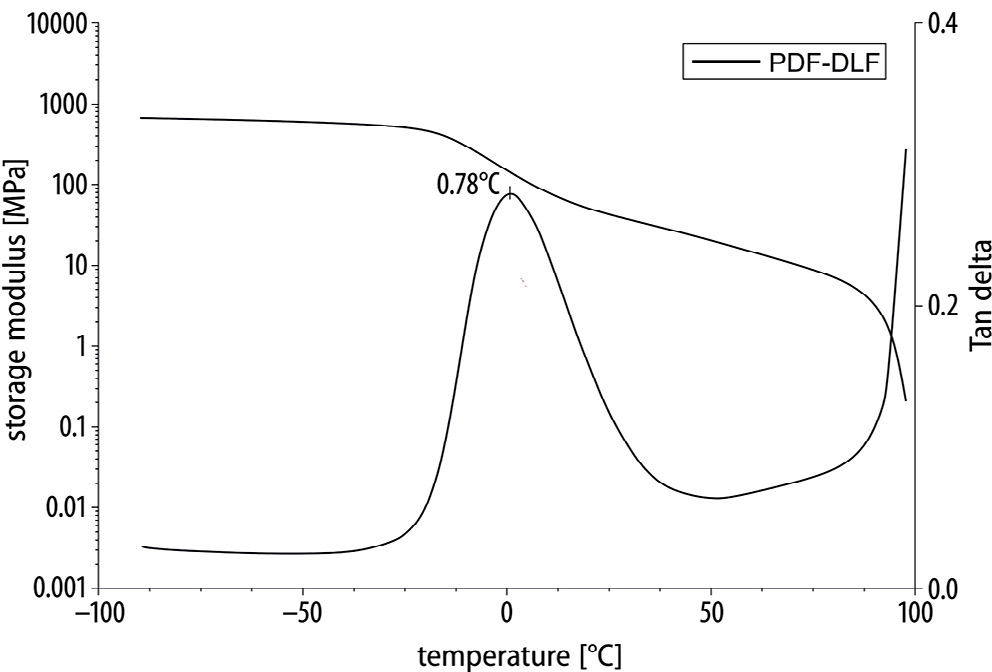
Dynamic mechanical thermal analysis was performed to assess the mechanical properties of the PDF-DLF material (Figure 2). The DMTA measurements indicate the viscoelastic characteristics of furan-based copolyesters, shedding light on their thermo-mechanical dynamics. Below the glass transition temperature (Tg), the copolyesters exhibited a consistently stable storage modulus. This stability indicates a solid and rigid nature within this temperature range, emphasizing the material’s ability to maintain structural integrity and stiffness. Upon reaching the Tg, an evident decline in the storage modulus values was observed. This transition denotes a shift in molecular mobility, typically associated with a reduction in stiffness. The material undergoes a transformation toward increased flexibility, accompanied by heightened molecular movement, influencing its mechanical behavior. This particular aspect is pertinent in applications where flexibility is a critical parameter. As the temperature continued to rise, the storage modulus gradually decreased until reaching the melting temperature (Tm). This gradual reduction in modulus values signified a transition from a more solid to a more liquid-like state.
Enzymatic degradation played a pivotal role in assessing the suitability of the PDF-DLF copolymer for biomedical applications. The material, subjected to lipase from P. cepacia
in a DPBS solution, aimed to simulate physiological conditions within the human body. Understanding how the polymer undergoes enzymatic breakdown was essential for ensuring its compatibility with biological systems.
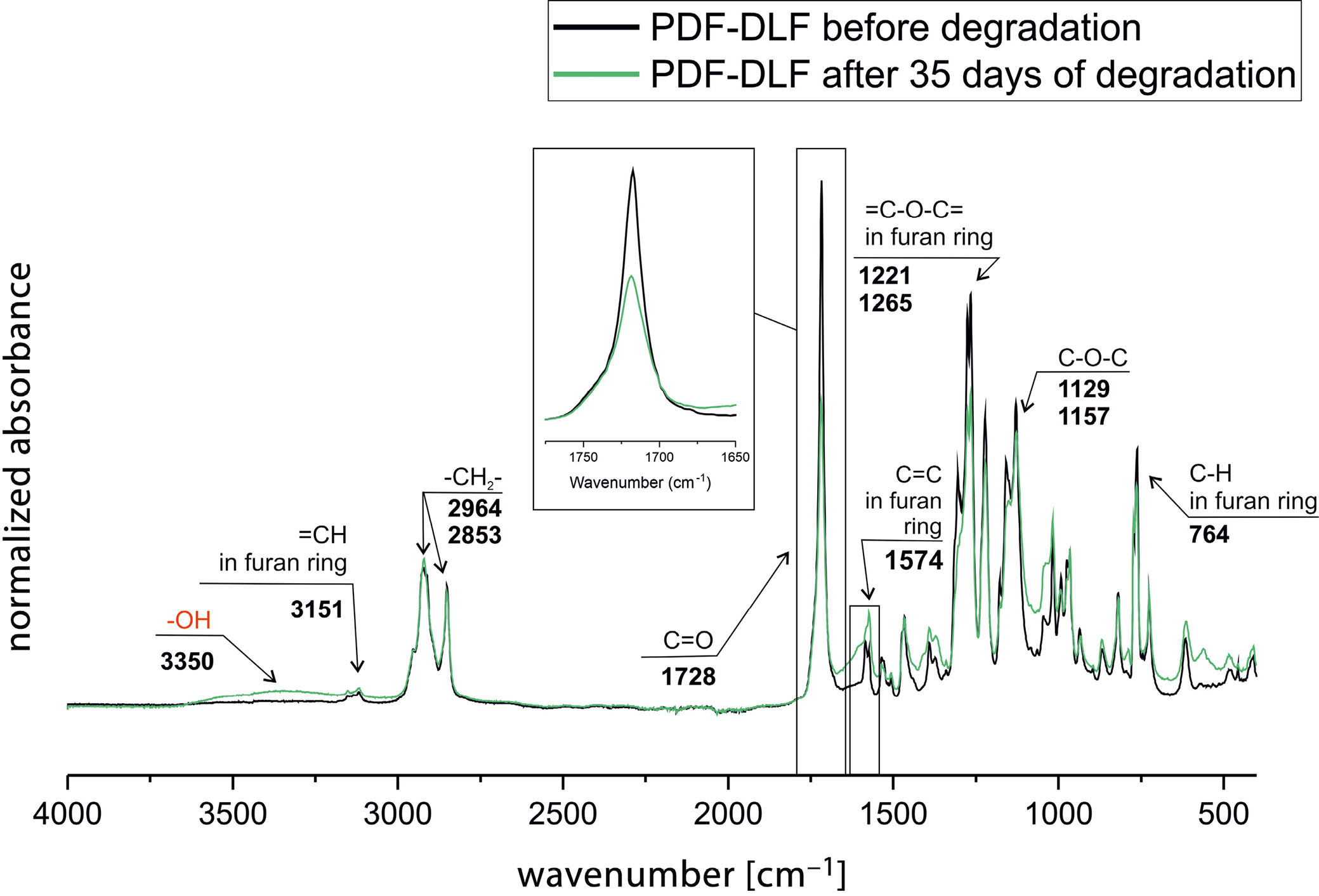
The FTIR analysis before and after degradation revealed changes in intensities and shifts in peaks of characteristic functional groups (Figure 3). A clear decrease in intensity is visible in the carbonyl group region (C=O), potentially indicating hydrolysis of ester bonds and monomer formation. This is further supported by the appearance of a peak arising from –OH groups. Additionally, changes are noted in the region where C=C bonds in the furan ring appear. These structural alterations suggest that the material undergoes disintegration under the influence of the enzyme.
To verify changes in the molecular weight, SEC measurements were conducted on materials both before and after 35 days of degradation. The observed molar mass changes (Table 2), along with the increased dispersity index, suggest that the material has undergone enzymatic degradation. The degradation of polyesters with semi-aromatic structures is typically known to be relatively inefficient. However, in this case, it appears that the enzyme is targeting the amorphous regions, specifically the DLF soft segments. The visible alterations in molecular weight serve as a clear indicator of ester bond cleavage. This phenomenon is significant as it highlights the susceptibility of specific regions to enzymatic attack, contributing to the molecular rearrangement and changes observed.
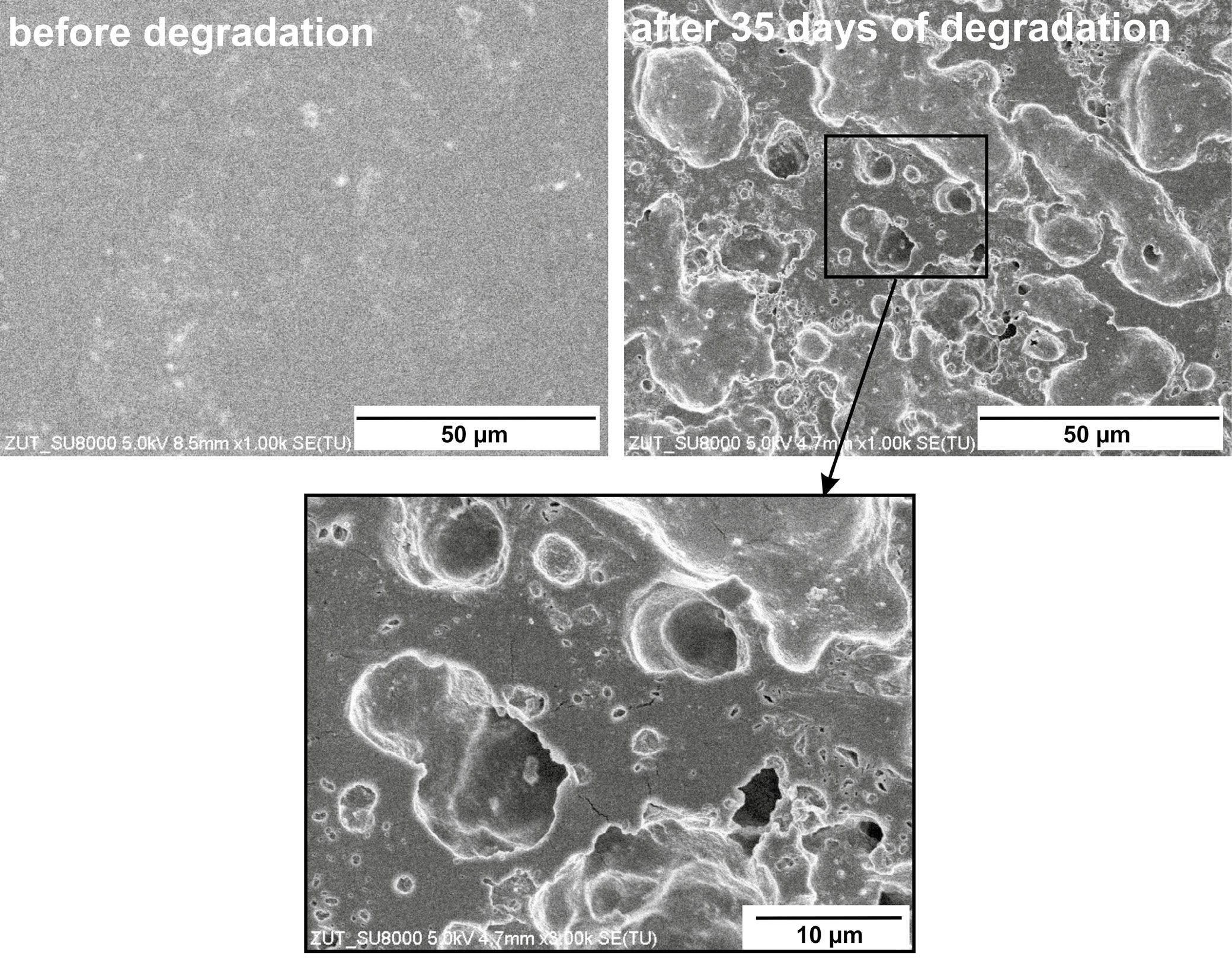
Interestingly, SEM analysis displayed noticeable surface changes, including breaks and holes following degradation. Figure 4 illustrates these alterations on the material surface, indicating a localized impact. The results suggest that enzymatic degradation likely occurs through a surface erosion mechanism rather than extensive bulk breakdown.
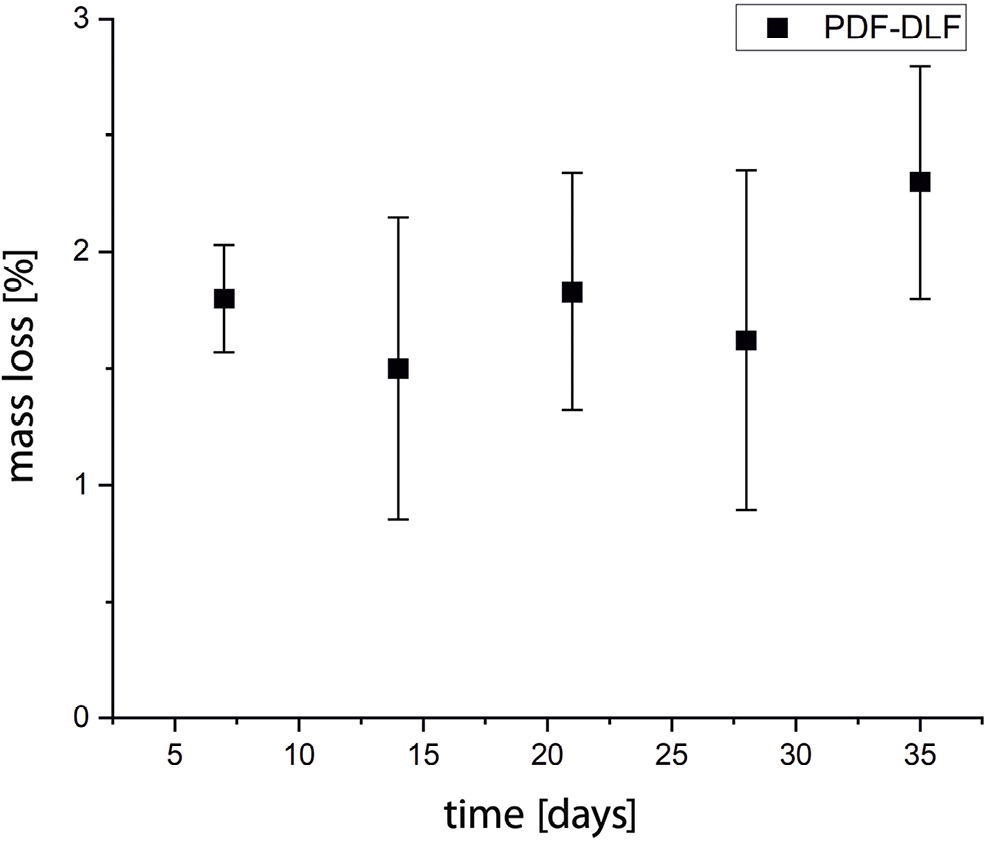
Finally, the detected mass loss of slightly over 2% during the 35 days indicated relatively slow enzymatic degradation (Figure 5). This behavior is typical of the first phase of enzyme-assisted chemical hydrolysis, and only after this is usually followed by metabolizing the fragments and rapid loss of polymer mass.
All these factors collectively suggest that PDF-DLF is susceptible to enzyme-assisted chemical hydrolysis, despite no significant mass loss. However, further in-depth investigation and research in this matter are necessary, potentially involving the use of different enzymes. For example, enzymatic degradation was also studied utilizing recombinant Thermobifida cellulosilytica cutinase 1 (Thc_Cut1), cutinase from Humicola insolens (HiC) and lipase from Porcine pancreas (LPP) on poly(ethylene furanoate) (PEF), revealing promising results and correlations.34, 35, 36, 37, 38 Thc_Cut1 exhibited higher activity on higher molecular weight PEF powders, releasing 13 mM of FDCA after 72 h.36 Crystallinity significantly impacted degradation rates, accelerating hydrolysis in cases of lower crystallinity. Conversely, HiC demonstrated greater activity than Thc_Cut1 on the highly crystalline forms of PEF, thereby slowing enzymatic hydrolysis.34
Moreover, a recent study by Kim et al. explored the enzymatic degradation of poly(butylene adipate)-co-(butylene furanoate) (PBAF) copolymers with varying furan ring fractions (50 mol% and 70 mol%) using Thermomyces lanuginosus lipase (TLL), a highly selective catalyst.39 The study revealed that PBAF with a 50 mol% furan ring fraction experienced a mass loss exceeding 80% within a few days, while PBAF with a 70 mol% furan ring fraction exhibited only around 1–2% mass loss over the same period. These findings align with our research, indicating that a higher proportion of furan units in the copolymer results in a slower rate of degradation. Hence, careful design considerations for such materials are essential to accelerate this process, particularly when attempting to meet the requirements for medical applications. All enzymes used for the enzymatic degradation studies acknowledged above were in their native form (not immobilized).
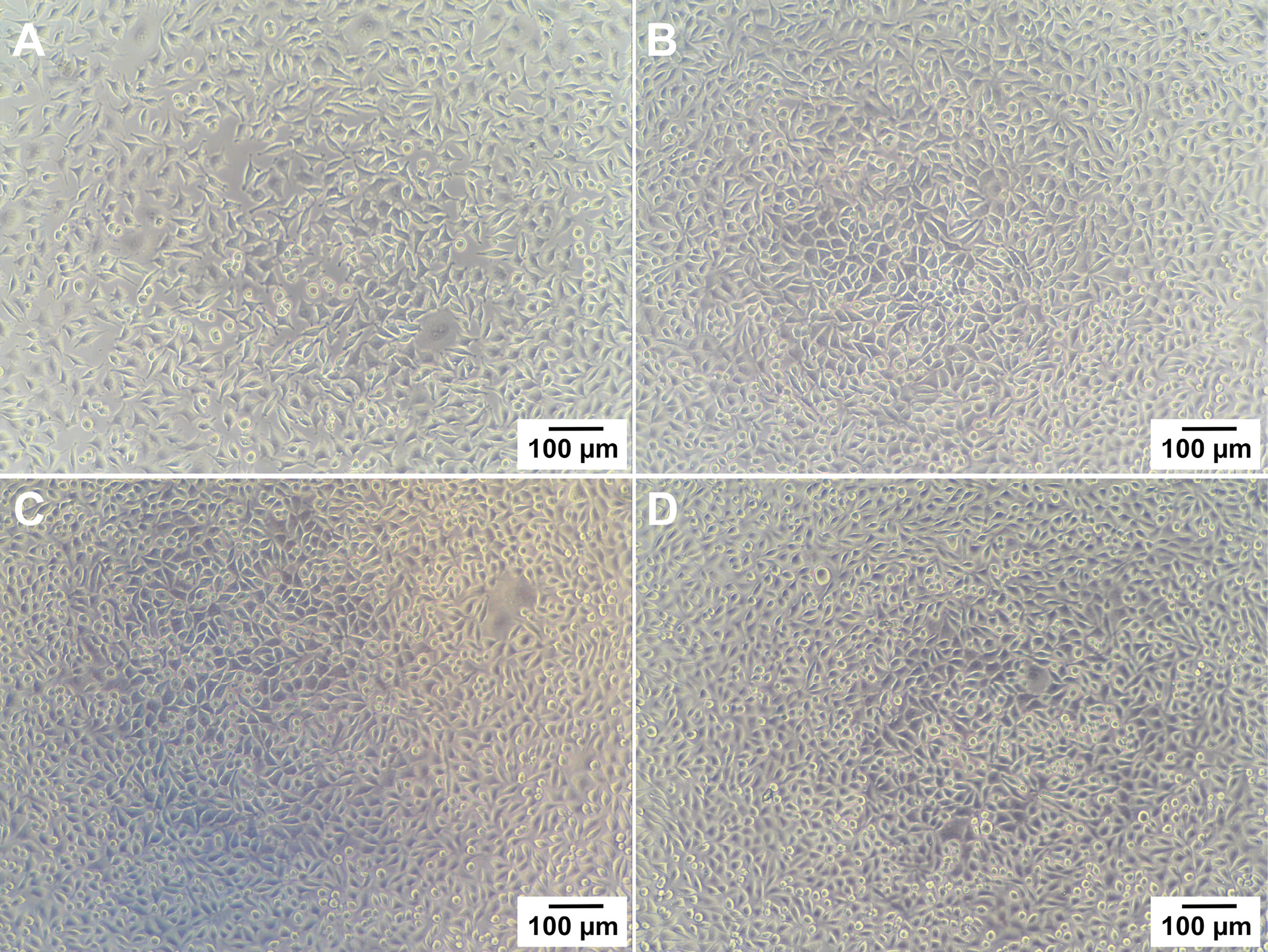
Given the potential application of the copolymer in the biomedical field, specifically in contact with the body, it became imperative to assess the material’s biocompatibility. To investigate any potential cytotoxic or growth-inhibitory effects of the PDF-DLF copolyester, an in vitro indirect contact assay was conducted using L929 murine fibroblasts. Over 24 h, cells were exposed to extracts from both the reference material (PCL) and the PDF-DLF samples, and cell viability was assessed with light microscopy and a resazurin viability assay. Following the 24-h incubation, noticeable adverse effects of both PCL and PDF-DLF materials were observed (Figure 6). Importantly, the absence of toxic contaminants was confirmed by the robust growth and typical cell morphology observed when cells were treated with extracts from control, reference and tested materials. The microscopic observations were supported by the results of the resazurin viability assay, from which normalized viability values were calculated. These values were in accordance with the visual evaluation. Considering the typical doubling time of L929 cells is approx. 20–22 h, viability values below 70% are indicative of cytotoxicity according to ISO10993. The average values of normalized cell viability (CV%) for PCL and PDF-DLF were 97 ±5% and 95 ±7%, respectively.
Ultimately, PDF-DLF underwent a preliminary study involving electrospinning to assess its processability and suitability for application in the field of tissue engineering as scaffolds.
In the course of our investigations, it was observed that polymer concentrations below 20% resulted in electro-spraying, revealing inadequate fiber formation, while a concentration of 50% led to the formation of a bulk structure. This was attributed to the solvent evaporation rate on crystalline phase formation of the polymer during electrospinning, which can be irrespective of polymer drawing forces.40
Subsequent experimental studies identified that the optimum polymer concentration of 30%, coupled with a 24-gauge needle, yielded the most favorable electrospinning outcomes. The fine-tuning process extended beyond concentration adjustment and encompassed modifications to key electrospinning parameters, including the distance between the needle tip and the collector, applied voltage and polymer solution flow rate. Through systematic adjustments, the following optimal parameters were identified; a distance of 20 cm, a flow rate of 5 mL/h and an applied voltage of 15 kV. The establishment of these conditions not only enabled the reproducibility of the electrospinning process but also underscored the importance of optimal parameters in governing the morphological characteristics of the resulting fibers.
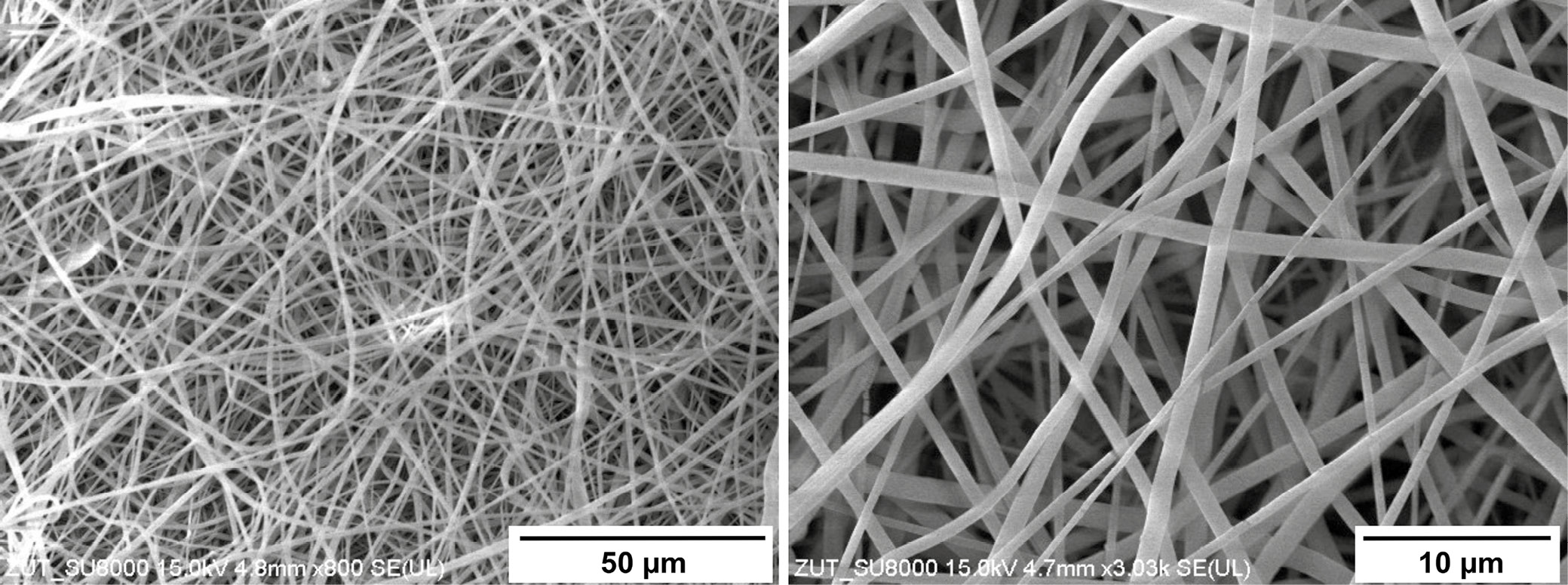
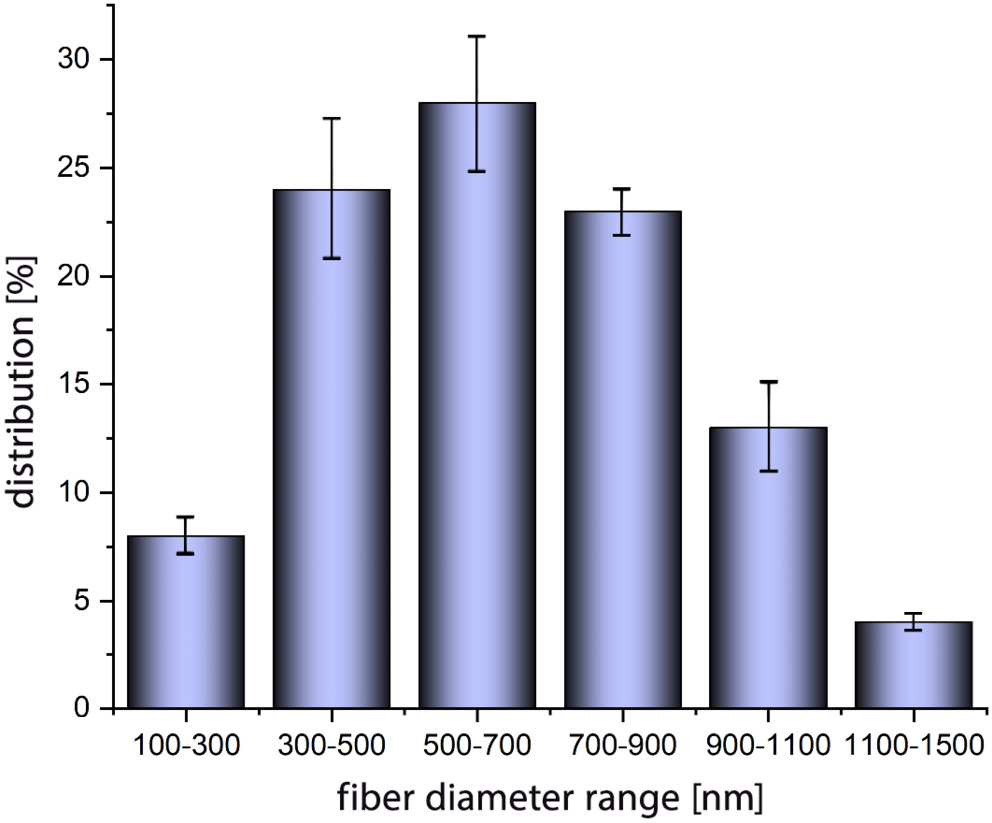
Figure 7 presents the SEM images of the obtained fibers through the optimized parameters of the electrospinning. The acquired fibers exhibit structural homogeneity and uniform distribution. Given the utilization of a flat aluminum collector during the electrospinning process, the resulting fibers exhibit a random orientation. Figure 8 presents a comprehensive analysis of fiber diameters and their distribution, conducted through ImageJ software (National Institutes of Health (NIH), Bethesda, USA). The findings reveal that, for the copolymer under investigation, the majority of fiber diameters fall within the range of 500–700 nm. This outcome underscores the electrospinning potential of the polymer, showcasing characteristics similar to the ECM.41, 42 The versatility of these fibers extends their applicability to diverse domains, including, but not only limited to, tissue engineering.
Conclusions
In response to the increasing demand for sustainable materials, particularly in the polyester domain, this research has been dedicated to the comprehensive evaluation of a furan-based copolymer, PDF-DLF. This copolymer, with a 70–30 wt% hard-to-soft segment ratio, was examined for its potential in medical applications, with a specific emphasis on its viability as a scaffolding material for tissue engineering.
The results underscore the significant steps made in the development of sustainable materials with versatile processing capabilities. PDF-DLF exhibited promising mechanical and thermal properties. It was successfully electrospun into nanofibers, with diameters ranging from 500 nm to 700 nm, holding promise for the creation of biodegradable scaffolds for medical engineering.
Furthermore, biocompatibility assessments revealed promising results. In vitro studies using L929 murine fibroblasts demonstrated a high cell viability, indicating the material’s compatibility and lack of cytotoxic effects. Enzymatic degradation studies revealed structural and molecular changes, as well as localized impact on the polymer’s surfaces. Although registered mass loss is not significant, it suggests material susceptibility to enzymatic degradation. However, while this study has unveiled promising aspects of PDF-DLF, further research is imperative. Deeper research on enzymatic degradation mechanisms, which could utilize different enzymes, is recommended.
In conclusion, the findings of this research not only contribute to the expanding knowledge of sustainable materials but also open new avenues for PDF-DLF in critical industries, particularly in the ever-evolving field of biomedical engineering. The convergence of biocompatibility, mechanical properties and processing capabilities in PDF-DLF suggests a multifaceted solution to environmental and engineering challenges, warranting continued exploration and refinement.