Abstract
Over the years, exopolysaccharides (EPSs) have been utilized in various areas of research, including health, industry, environment, and agriculture, due to their flexible physical, chemical and structural properties that can be readily modified to suit desired purposes. Current research trends have shown that EPS production is dependent on numerous factors which can be combined to varying extent to optimize production yields. Although the majority of research is directed towards their industrial and medicinal uses, these chemical substances possess peculiar characteristics which are also exploited for biomedical research, where they are being used as drug delivery systems, some of which include their abundance in nature, biocompatibility, biodegradability, non-toxicity, and ability to efficiently encapsulate sensitive bioactive agents. However, despite the numerous beneficial prospects of microbial EPSs in drug delivery, there are limitations to the commercial production and industrial applications of these biopolymers. These limitations have inspired revolutionary research into the cost-effective production of safe EPSs polymers. In this review, we classify EPSs and discuss their methods of extraction and characterization. We also summarized current drug delivery applications and discussed limitations to extensive industrial commercialization of EPSs, while highlighting prospects for the utilization of microbial EPSs and implications for research.
Key words: exopolysaccharides, drug delivery, biopolymers
Introduction
Exopolysaccharides
Exopolysaccharides (EPS) are extracellular metabolic by-products excreted by bacteria, yeast, fungi, etc.1 They are high-molecular-weight polymeric biomolecules with glycosidic linkages connecting monosaccharide units that are synthesized by microbial cells and secreted to the outside of the cells, where they perform beneficial functions, including protection from extreme pH and temperature.2 Exopolysaccharides are crucial components that determine the structural and functional integrity of biofilms, which provide microorganisms with protection from harsh environments.3, 4 For example, xanthan, alginate and cellulose form a biofilm on bacterial cell surfaces that serves as a protective cover.5 Along with these biological functions, EPSs have distinct chemical and physical properties that make them suitable industrial raw materials.6
In agriculture, EPSs have been used to improve the flow of pesticides and uniformly disperse solid components in formulations; the improved rheology of the formulation also ensures surface cling.7 Exopolysaccharides are also used as gelling agents for culture media, paints and ink, and detergents.
Pharmacologically, EPSs have shown peculiar biological bioactivities which have found therapeutic applications in animal and human medicine.8 These activities have demonstrated potential for use in prophylactic and antibiotic therapies. Some of them include; anti-inflammatory activities, antimicrobial and anti-tumor activities, hypocholesterolemic activities, immunomodulatory activities, and anti-diabetic activities.9, 10 Through technological advancement in immunological studies, EPSs have been used to develop capsular polysaccharide-based vaccines for infectious illnesses.11 Microbial EPSs have been extensively studied for their applications in vaccine development. They show prospects as antigen delivery systems and as antigenic materials (as adjuvants) to provide more robust immune responses.12
Due to their non-toxic nature, EPSs are being used to refashion international treatment models by modifying drug therapy, surgical therapy and disease diagnosis; this forms the basis of their biomedical applications.13 They are versatile biomedical applications because they have flexible physical, chemical and structural properties that can be readily modified to suit desired purposes. Furthermore, they have published and standardized methods of fermentation and isolation, which makes them suitable to meet global demands. As a result, EPSs have found numerous applications in tissue engineering and drug delivery. They have also been used as surgical sealants and coating materials for medical devices.14
The most notable biomedical application of EPSs is in the delivery of sensitive drug molecules and biomolecules. Exopolysaccharides have shown potential as drug delivery materials due to their capacity to effectively entrap bioactive agents. Through this application, they are used to improve the therapeutic efficacy of drugs and increase the drug shelf life and duration of pharmacological action.14, 15 The versatile applications of EPSs in drug delivery applications are owed to their abundance in nature, biocompatibility, biodegradability, and non-toxicity.1
Classifications and structure of exopolysaccharides
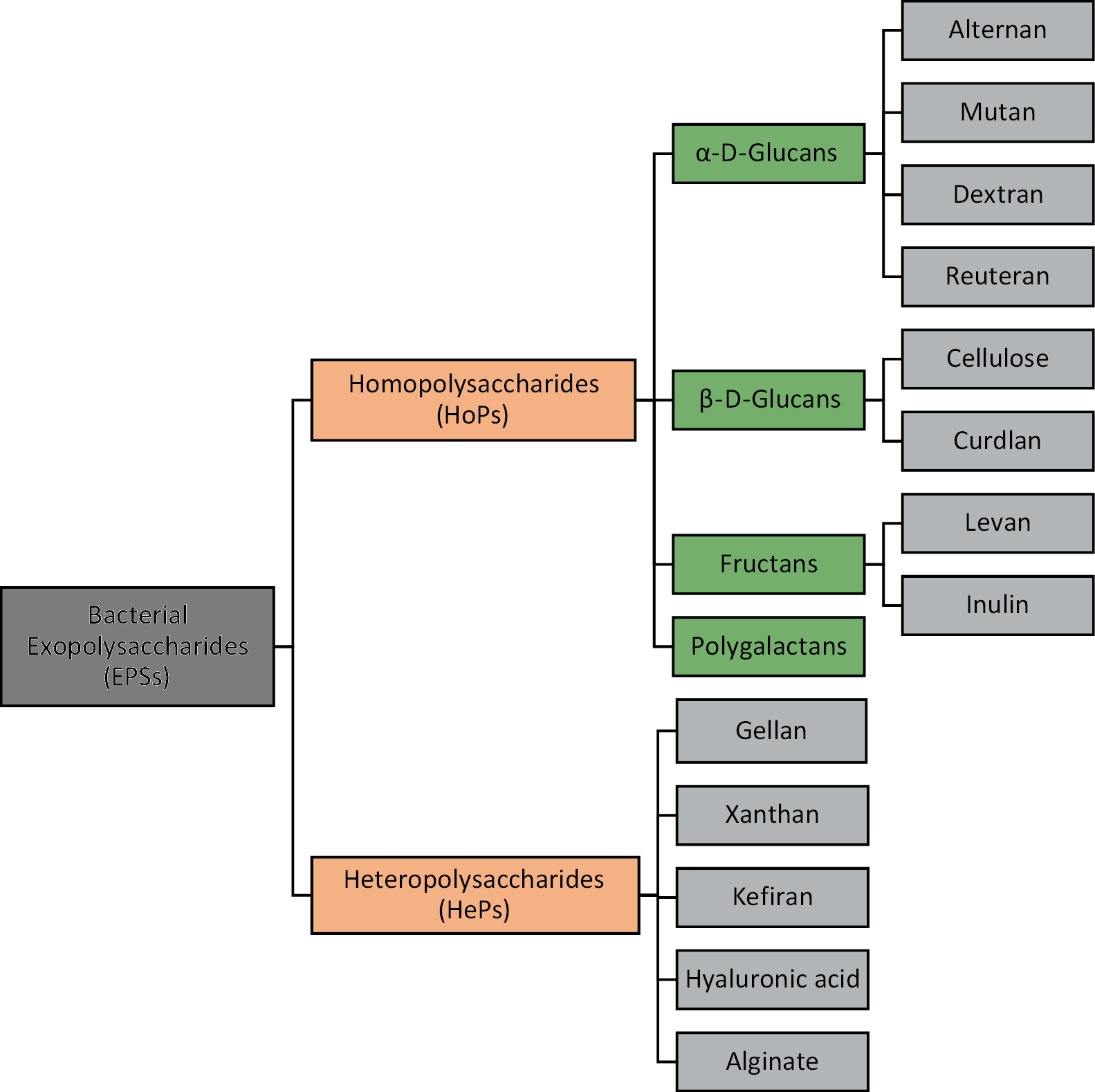
Due to differences in their chemical components which influence their physical, biological, chemical and functional properties, EPSs are a diverse and complex class of biomolecules.6 Based on their chemical composition, EPSs are primarily categorized according to their monomeric content into homopolysaccharides (HoPs), which contain one type of sugar monomer unit, and (HePs) which have several recurring subunits of different sugars from disaccharides to heptasaccharides, monosaccharide derivatives and/or substitutes.16, 17 This classification is summarized in Figure 1.
To make further distinctions within the major categorizations, EPSs are subclassified based on the nature of their monomeric units, linkages and the degree of branching.16
Homopolysaccharides
Homopolysaccharides are identified based on monosaccharide units and classified as glucans, fructans and galactans, according to the presence of sugars (D-glucose, D-fructose and D-galactose) in their backbones.17, 18 Glucans exhibit varying degrees of branching and monomeric linkages; they are further classified into 1) α-D-glucans (alternan, reuteran, dextran, and mutan), where D-glucose residues are linearly or non-linearly interlinked by α-l-2, α-l-3, α-l-4 and/or α-l-6 glycosidic bonds; and 2) β-D-glucans (cellulose and curdlan), comprising of D-glucose connected by β-l-3 bonds and β-l-2 branching.6 Due to these different linkage patterns, glucans exhibit varying degrees of water solubility and viscosity. Fructans are water-soluble and are grouped into 1) levan with β-2-6 bonds and 2) inulin with β-2-1 bonds.19 Galactans, although less abundant, are also water-soluble and consist of α-1-6 linked galactose monomers.17
Heteropolysaccharides
Heteropolysaccharides comprise repeating sugar units at varying ratios which may include D-xylose, D-ribose and D-arabinose, which are pentose sugars, and D-galactose, D-mannose and D-glucose, which are hexose sugars. The HePs also include uronic acids such as D-galacturonic acids and N-glucuronic acid. Other HePs are acetylated monosaccharide units, including N-acetyl-glucosamine and N-acetyl-galactosamine.20, 21 They may also be branched or unbranched, and contain non-sugar substituents (organic or inorganic) such as phosphate, acetyl and glycerol.22 The linkages between monomeric units in polymers are rigid 1,2-α- or 1,6-α- and 1,4-β- or 1,3-β- linkages, which are more flexible.16 Examples include gellan, xanthan, kefiran, hyaluronic acid, and alginate.1, 18
Exopolysaccharides can also be identified by their degree of association with the microbial cell wall and where their synthesis occurs. Based on the degree of attachment, they can be broadly grouped as: 1) capsular EPSs, which are firmly attached to the cell surface, creating a resistance mechanism against attacks from phagocytes and bacteriophages, and protection against excessive water loss and osmotic stress; and 2) slime EPSs, which are loosely associated with the cell surface and can be easily isolated. Depending on the location of synthesis, extracellular EPSs are produced outside the cell envelope, whereas intracellular polysaccharides are accumulated inside the cells and released outside the cells.1, 23
Microorganisms producing exopolysaccharides
Microorganisms that produce EPSs are abundant in nature and have been identified in numerous environments in the ecosystem, especially those with increased carbon–nitrogen ratios.24, 25 Exopolysaccharides can therefore be extracted from bacterial, fungal and archaebacterial sources (such as algae).13, 26
Some bacteria genera that produce EPSs are Acetobacter (cellulose), Agrobacterium (curdlan), Bacillus (levan), Brenneria, Geobacillus, Gluconacetobacter (cellulose, levan), Halomonas (levan), Lactobacillus (dextran, kefiran), Rhizobium (curdlan), Saccharomyces, Sarcina, Pseudomonas (alginate, gellan, cellulose, gellan), Streptococcus (levan, hyaluronic acid), Xanthomonas (xanthan), and Zymomonas (levan).18, 27 Some probiotic bacteria, such as Lactobacillus (dextran), Leuconostoc (dextran), Lactococcus, Bifidobacterium (hyaluronic acid), Streptococcus (levan, hyaluronic acid), and Enterococcus have also been used to synthesize EPSs for numerous medical and biomedical applications.1, 14 Other bacterial sources include marine bacteria such as Vibrio diabolicus (hyaluronian-like EPS) and Arthrospira platensis, and extremophiles such as Bacillus thermantarcticus and Geobacillu sthermodenitrifican.13, 16
Due to their cellular and structural complexity, fungi are considered to be major non-bacterial sources of EPSs. Major fungi from which EPSs are isolated include Aspergillus fumigatus (galactosaminogalactan), Candida albicans (chitosan, chitin) and Zygosaccharomyces rouxii (chitosan, chitin). Other examples of EPS-producing fungi are Botryosphaeria sp. (botryosphaeran), Pleurotus ostreatus (pleuran), Aureobasidium pullulans (pullulan), Schizophyllum commun (schizophyllan), Phellinus linteus, Ganoderma lucidium, Fusarium sp., and Inonotus obliquus.13 Exopolysaccharides have also been extracted from surface biofilms of archaea that grow at extreme temperatures and salt concentrations: Thermococcus, Sulfolobus, Archaeglobus fulgidus, and Thermococcus litoralis.28
Production of exopolysaccharides
Biosynthesis of exopolysaccharides
Among various species of EPS-producing microorganisms, the biosynthetic pathways involved in EPS biosynthesis are notably similar, regardless of their different characteristics.6 In bacteria, the synthesis of EPS occurs through intricate pathways that consist of specialized enzymes, carriers and transporter proteins29: They are the intracellular and extracellular pathways.1 Depending on their natures, bacteria EPSs can either be produced entirely outside by extracellular bacterial enzymes, or synthesized inside the cells and released into the cell surrounding.30
Through the intracellular biosynthetic pathway, extracellular sugar residues are taken into the cells, broken down into various monomers, polymerized and transported out of the cells through a membrane-bound lipid carrier protein.31 The extracellular biosynthetic pathway occurs outside the cell when sugar molecules are lysed into uniform monomeric units which are assembled, with glycosyl transferases enzymes or fructosyl transferases, into polymers that are secreted into the surrounding environment.1, 19 Although the extracellular pathway is majorly utilized in the production of HoPs, either of both biosynthetic pathways are involved in the synthesis of HePs.32, 33
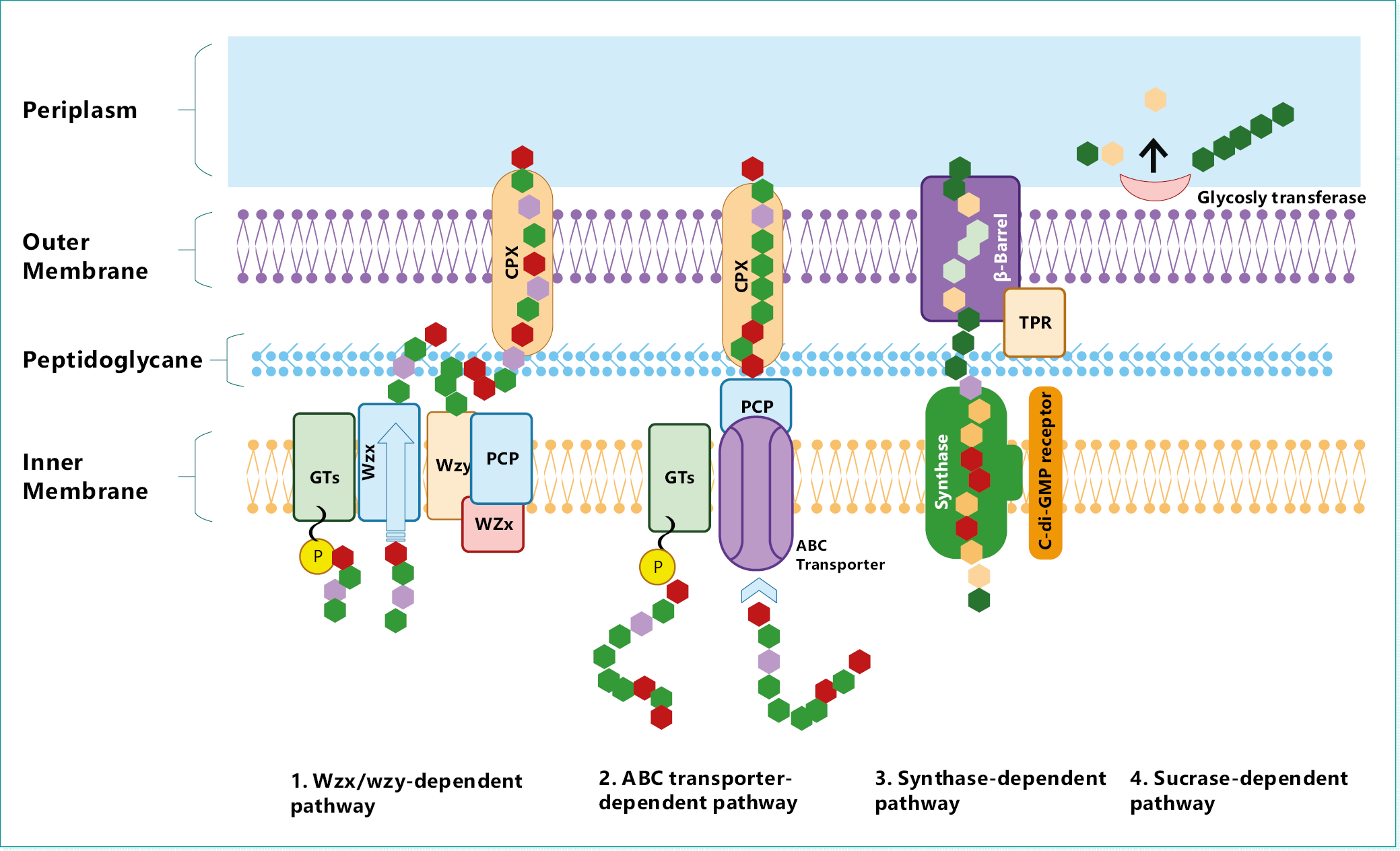
In general, there are 4 mechanisms involved in the biosynthesis of EPSs in bacterial cells14: the Wzx/Wzy- dependent pathway; the ABC transporter-dependent pathway; the synthase-dependent pathway; and extracellular biosynthesis by sucrase protein.34, 35, 36 These mechanisms are illustrated in Figure 2.
1. Wzy/Wzy-dependent pathway: At the inner membrane, imported monomeric sugar molecules are converted into sugar nucleotides and linked to a lipid carrier.37 Via the addition of more sugar units by glycosyltransferases, the sugar chain is extended and transported, as an oligosaccharide unit, through the membrane by Wzx flippase.38 The unit is them enzymatically modified, and the Wzy protein polymerizes the altered oligosaccharide into polysaccharides, which are released into the cell exterior by outer membrane polysaccharide transporters.14, 39
2. ABC transporter dependent pathway: This pathway is employed in the synthesis of capsular EPSs40 and it is similar to the Wzy/Wzy-pathway but excludes the activities of Wzx flippase and Wzy protein. Polysaccharide chains synthesized by glycosyltransferases from nucleotide sugar units are transported across the inner membrane via a tripartite efflux pump complex – the (ATP)-binding cassette (ABC) transporter.41
3. Synthase-dependent pathway: In this pathway, same-type monosaccharide units are arranged into undecaprenyl phosphate glucose units by synthase protein located in the membrane.42
4. Extracellular synthesis by sucrase enzyme: This extracellular pathway occurs in the periplasm where sucrose is converted, outside the outer membrane, into its monosaccharide components (glucose or fructose) by sucrase enzymes. The monomer units are polymerized (with varying degrees of branching) by glycosyltransferases to form glucans or fructans – glucose and fructose units, respectively.14, 43
Heteropolysaccharides are mainly produced through the ABC transporter-dependent and Wzx/Wzy-dependent pathways, while HoPs – via the extracellular production and synthase-based pathways.1
Fermentation of microorganisms: Production media and incubation conditions
The quality and quantity of EPSs are determined by several factors; bacteria strains, nutrient level and makeup of the growth/production media (salt concentration and the carbon:nitrogen composition) and incubation conditions (such as time, pH and temperature).14, 44 Consequently, EPS production is subject to numerous non-microbial factors which can be combined and permutated to varying degrees to achieve different production yields and characteristics of EPSs. This complicates the selection process of production media and incubation conditions for EPS production, inspiring the research into predicting the best combinations and arrangement of culture conditions (variables) to maximize production yields using one-factor-at-a-time (OFAT) optimizations and statistical experiment designs such as response surface methodology (RSM).44, 45 The OFAT optimizations involve testing each variable, one at a time while holding other variables constant, to determine which variable has the most effect on EPS production. The RSM statistically analyzes several independent variables to predict their relationship with each other and their effects on production yield45; it is being extensively employed by researchers to optimize the production of EPSs in microbes using experimental designs including Plackett–Burman Design (PBD), Box–Behnken Design (BBD) and Central Composite Design (CCD).46, 47 Table 1 summarizes some findings of recent research works on the optimization of production factors to improve EPS production from microbial sources.44, 45, 46, 48, 49, 50, 51
Extraction and recovery of exopolysaccharides
The recovery process of EPSs includes concentration, isolation and purification. After EPSs are concentrated in the fermentation broths, they are isolated from microorganisms by centrifugation.45 More extreme methods are boiling in saline solution, sonication and autoclaving. Isolated EPSs are mostly extracted from isolated mixtures by solvent precipitation via the addition of a polar organic (such as ethanol) that reduces the solubility of EPSs in water.45, 46 Desalting and deproteination treatments may be further carried out to purify precipitated polysaccharides. The resultant precipitate is collected by centrifugation, filtration or sedimentation and dried under pressure.24, 25
To further separate sugars and minute proteins, the collected precipitate may undergo membrane filtration after dissolution in water. The filtrate is then freeze-dried to get the EPS powder which is packaged for use. 17, 52, 53 Anion exchange chromatography and size exclusion anion exchange are additional steps of purification employed in advance separation processes.54, 55
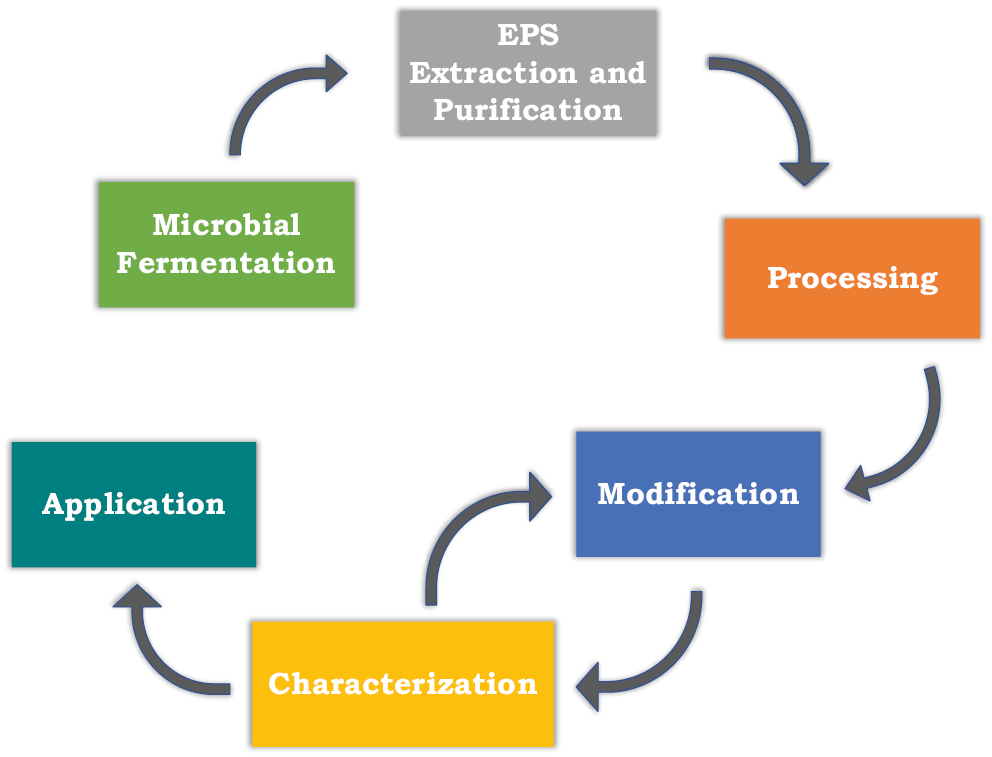
Oftentimes, extracted EPSs are utilized in the same form as they are extracted (native form). They may also be physically or chemically treated before their use.24 These treatments are applied as a means to modify the characteristics of these polysaccharides, thereby optimizing their suitability for pharmaceutical, food and biomedical purposes (Figure 3).
Characterization of exopolysaccharides
Extensive characterization of extracted EPSs requires the evaluation of monosaccharide content, ring formations, extent of branching, and molecular weight. However, because these parameters cannot be accurately evaluated with singular methods, multiple methods and techniques are employed in combination.6 Some of these parameters and respective determination methods are summarized in Table 2.34, 44, 45, 48, 49, 56, 57
Drug delivery applications of exopolysaccharides
The EPSs have shown potential as drug delivery materials for the delivery of sensitive drug molecules and biomolecules due to their bioactive function and capacity to hold bioactive agents effectively. Although they have similar functions as drug delivery systems, they are easier to apply as drug carriers than to produce as biological scaffolds of viable cells. This is because they can be readily modified to improve therapeutic efficacy by controlling and targeting the release of drugs in body tissues and protecting drug molecules from harsh physical and biological environments to increase the shelf life and duration of pharmacological action.14, 15 In addition to their abundance in nature, biocompatibility, biodegradability and non-toxicity are the main factors responsible for the ubiquitous drug delivery applications of EPSs.1
Nano- and micro-delivery systems
The development of nano- and microparticles is arguably the most significant application of EPSs in the delivery of drugs. The benefits of these include increased efficacy and decreased toxicity of encapsulated drugs. Levan nanoparticles exhibit decreased intestinal toxicity of selenium, iron and cobalt.58 Serum bovine encapsulated by Sezer et al. exhibited zero-order drug release kinetics.59 Hydrogel nanoparticles of curdlan derivatives have shown potential in the delivery of anticancer drugs.60 Because EPSs are bio-compatible and non-toxic, they offer more benefits in the green synthesis of silver nanoparticles compared to synthetic polymers.61
Transdermal drug delivery systems
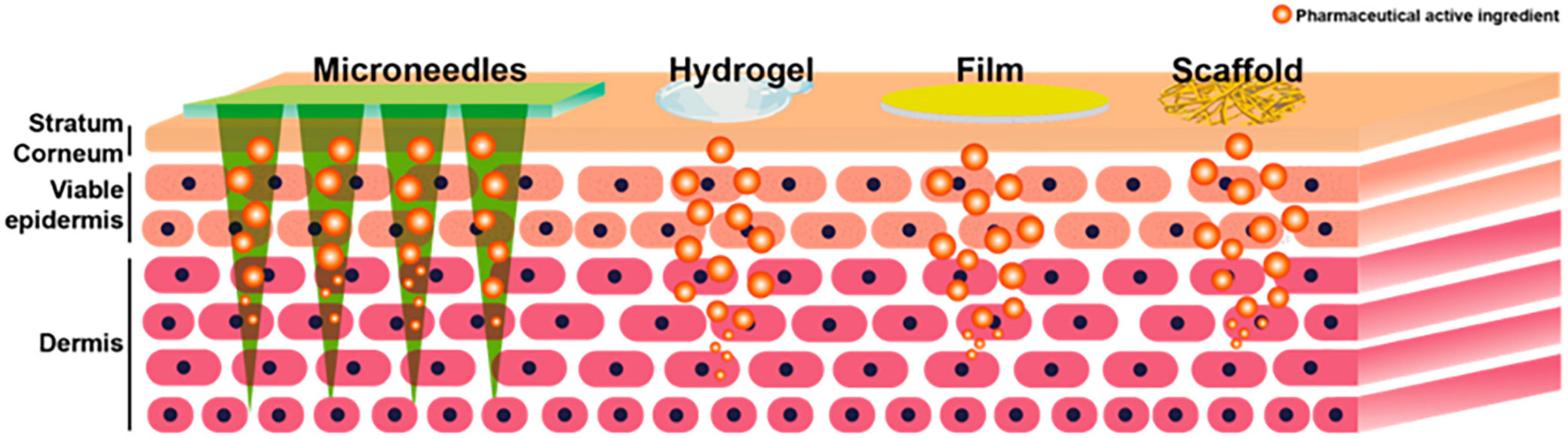
Exopolysaccharides are also utilized in the development of transdermal drug delivery systems (TDDS). Researchers postulate hyaluronic acid is the most commonly used marine EPS drug delivery via the transdermal route.62 Alginate is also frequently applied in TDDS to prepare microneedles (which have been used to deliver vaccines, bovine serum proteins and insulin) and nanoparticles (in combination with chitosan) for target antibiotic therapy of cutaneous pathogens.63, 64, 65 Transdermal drug delivery systems made with EPSs are constantly evolving. Recent TDDSs which are illustrated in Figure 4 vehicles include66:
1. Hydrogels: These are hydrophilic, polymeric networks which are 3-dimensional and have large water-holding capacities. A notable use is “smart” hydrogels that regulate molecule release by responding to changes in external stimuli.66
2. Films: These are thought to be a good substitute for topical patches and traditional topical dosage forms due to their flexibility, transparency, non-occlusivity, and ease of use because they are non-invasive. They also demonstrate higher drug loading capacity and prolonged drug release and drug retention on the skin.67, 68 The film-forming ability of EPSs makes them particularly suitable candidates in the formulation of these delivery vehicles.
3. Microneedles: These are efficient, minimally invasive vehicles of transdermal drug delivery, which typically create micro-channels through the layers of the skin and release the drugs, thereby localizing drug delivery and drug effects.69 Biodegradable polymers such as hyaluronic acid, alginate and cellulose are more beneficial as microneedle components than silicon and metals because they are non-biodegradable.70
4. Tissue scaffolds: Polysaccharides are used to enhance the mechanism of drug release and improve the performance of tissue scaffolds. Hydrogels are frequently used as tissue engineering scaffolds, and those based on alginate exhibit better mechanical strength and controlled drug release.71
Modifications to improve drug delivery
Bacterial EPSs can be modified in a variety of ways to customize drug delivery properties and enhance the functionality of the delivery systems. Altering the side chain and functional group composition via chemical reactions of cross-linking reactions is one way of modification.72 Partial sulfation of curdlan at the O-6 portion improves the aqueous solubility of curdlan hydrogel nanoparticles; however, the extent of substitution affects the thermal stability and ability to cross-link with polycytidylic acid.60, 73 Exopolysaccharides are also combined with other polysaccharides, biopolymers or synthetic polymers to optimize desirable material characteristics of these polymers and improve upon their limitations. This combination results in polymer blends with high-performance characteristics capable of being tailored toward various novel drug delivery applications. A study by Inoue et al. demonstrated the ability of cellulose membrane to increase the release of chlorhexidine (which complexed with β-cyclodextrin) by 10-fold; this was postulated to be a result of rigid chemical interactions between cellulose and the chlorhexidine-β-cyclodextrin complex.74 In vitro studies of ciprofloxacin microspheres demonstrated adequate gastric protection of ciprofloxacin by kefiran-alginate polymer blends.75 Similarly, xanthan hydrogels modified with succinic anhydride extended the release of gentamycin over 9 days.76
Future prospects for the use of microbial exopolysaccharides
The spectrum of future EPS applications is as wide as the extent to which their functionality can be improved. Consequently, EPS-based drug delivery systems show promise in revolutionizing the paradigm of drug delivery.12 Diverse works of research have been dedicated to unearthing the untapped drug delivery potentials of EPSs.
Temperature- and pH-sensitive formulations
In transdermal delivery systems, they are capable of being modified with nanotechnology to prepare “smart” formulations which release entrapped drug molecules in response to temperature and pH changes at target delivery sites.66 Skin conditions induced by pH disparity have been treated with isoliquiritigenin-loaded hydroxyethyl cellulose – hyaluronic acid pH-sensitive hydrogels.77 In combination, hydrogels sensitive to temperature and pH have been developed; gallic acid loaded within Pluronic® F-127 bi-responsive hydrogels of chitosan oligosaccharide lactate and hyaluronic acid nano-conjugates have been used to resolve atopic dermatitis.78 In addition to the potential of improved clinical therapy in preventing infections and improving wound healing, these “smart” formulations have the benefit of being cost-effective.66
Simultaneously, in systemic delivery systems, EPSs show promise as substrates for “Intelligent Drug Delivery Systems”.79 The fungal EPS pullulan which, due to its recurring hydroxyl groups, is especially amenable to derivatization through chemical means, has been grafted on the backbones of poly-(N-isopropyl-acrylamide)-co-acrylamide and succinic carboxyl ether groups in modulating the release of lysozyme protein using pH and temperature.80 Similarly, carboxy-methyl pullulan modified with polyether-amine has demonstrated sol–gel transition at physiological temperature, providing assurance for thermally regulated sustained drug delivery.81
From current research in drug delivery, microbial EPSs show potential for application in targeted gastrointestinal delivery to the intestines. A typically useful feature of EPSs is their swelling and shrinking tendencies in response to changes in their environment; swelling increases the porosity of the polymers, thus triggering the release of contained drugs. Due to this sensitivity, restriction of drug release to the upper section of the small intestine is possible. This is because the majority of EPSs that are sensitive to pH shrink in mediums with low pH and swell in environments with high pH.82 Therefore, such oral delivery systems withhold the release of the drugs till the upper intestine, where swelling occurs due to the high pH.83 Exopolysaccharides are also being modified to improve target drug release even in an acidic medium. Similarly, non-starch EPSs that are highly stable and resistant to digestion all through the gastrointestinal tract (GIT) remain intact till the colon, where the colonic bacterial flora degrades them. This implies a possibility for target drug delivery to the colon.84
Interpenetrating polymer network
Exopolysaccharide hydrogels may also have the potential for molecular-scale penetration and drug delivery.12 This is demonstrated by the entrapment and the extended-release properties shown by the interpenetrating polymer network (IPN) of alginate and polymers with an anti-inflammatory agent (indomethacin), an antibiotic (gatifloxacin), anticancer agent (5-fluorouracil), and an anticoagulant (heparin).85 Steroid hormones are bioactive agents that stand to benefit significantly from this potential, as they are hardly mentioned in recent EPS research. Micro- and nano-transdermal delivery systems can bypass the side effects associated with their oral administration to improve their therapeutic efficacy.12
Discussion
Despite the numerous beneficial prospects of microbial EPSs and their composites in drug delivery, there are limitations to the commercial production and industrial applications. The most apparent of these limitations is the high cost associated with production and extraction, which is non-commensurate with their commercial value.16 This is consequent to the complexities of their cost-intensive fermentation, isolation, purification, and characterization processes; reconciliation of these with the often-minimal production output of EPSs shows the poor economic feasibility and sustainability of large-scale EPS production. Another limitation is the relative paucity of knowledge about the interconnection between structure and biological functions.9 This is particularly complicated for bacterial EPS by the realization that the structure and biological effects of EPSs vary with every strain of bacteria.14 There is also the concern of health risks associated with certain microbial sources. For instance, the most studied microbial source of alginate is Pseudomonas aeruginosa, a bacteria linked to numerous infections with severity ranging from mild to severe. Although genetically engineered P. aeruginosa strains that are not pathogenic have been developed by researchers such as Valentine et al.,86 there is still a significant proportion of un-engineered microbial sources. Bacterial capsular EPSs have been found to possess surface antigens that elicit immunogenic responses. This contributes to the pathogenicity risks of microbial EPSs.87 Therefore, it would not be quixotic to envision a revolution in the production processes of EPSs as a prospect of microbial EPSs. The main objective of the revolution would be to enhance commercialization by optimizing the production yield and quality of microbial EPSs while minimizing their cost of production. There should be more research studies on approaches to achieve this, such as methods to optimize industrial-scale fermentation and extraction processes,14 as well as on developing methods of artificial chemical synthesis to aid the modification and enhancement of structural and functional properties of EPSs,88 utilization of less expensive production materials (living and non-living), development of manipulation methods (mutagenesis, genetic and metabolic) of producing microbial strains with higher yields,38 and less pathogenicity. All these will ultimately result in a significant improvement in the commercial value of these biomaterials and, consequently, their availability for various industrial and biomedical applications.
Specifically, these developments promise a significant impact in Africa by improving the current perspective on the utilization potentials of EPSs. A random preliminary study by Osemwegie et al. to evaluate the course of global research on EPSs over a period of 18 years was used to provide an overview of African research on EPS applications. The review showed that Africa ranked low among the continents with prominent research in microbial EPS research and application. These authors related the small number of publications to the low interest of African countries in the numerous industrial benefits of EPSs despite the possibility of Africa being one of the best repositories of EPSs in the world.13
Conclusions
As the commercial value and industrial applications of microbial EPSs increase, there is sure to be a corresponding paradigm shift in African research on the potential applications and benefits of these biomaterials.