Abstract
Background. Many substances are used to increase the viscosity of eye drops and reduce their surface tension. Their function is to prolong the persistence of the product on the surface of the eyeball and to increase the bioavailability of the pharmacologically active ingredient.
Objectives. To investigate the surface tension of substances added to the eye drops, with the main aim of modulating properties of the preparation.
Materials and methods. Five substances contained in solutions proposed for the development of eye drops were studied: sodium hyaluronate macromolecular (H-Na W), sodium hyaluronate ultramolecular (H-Na UM), hyaluronic acid 4% (K-H), methylcellulose (MC), and polyacrylic acid (PA). The main method was to study the surface tension using the du Noüy ring tensiometer.
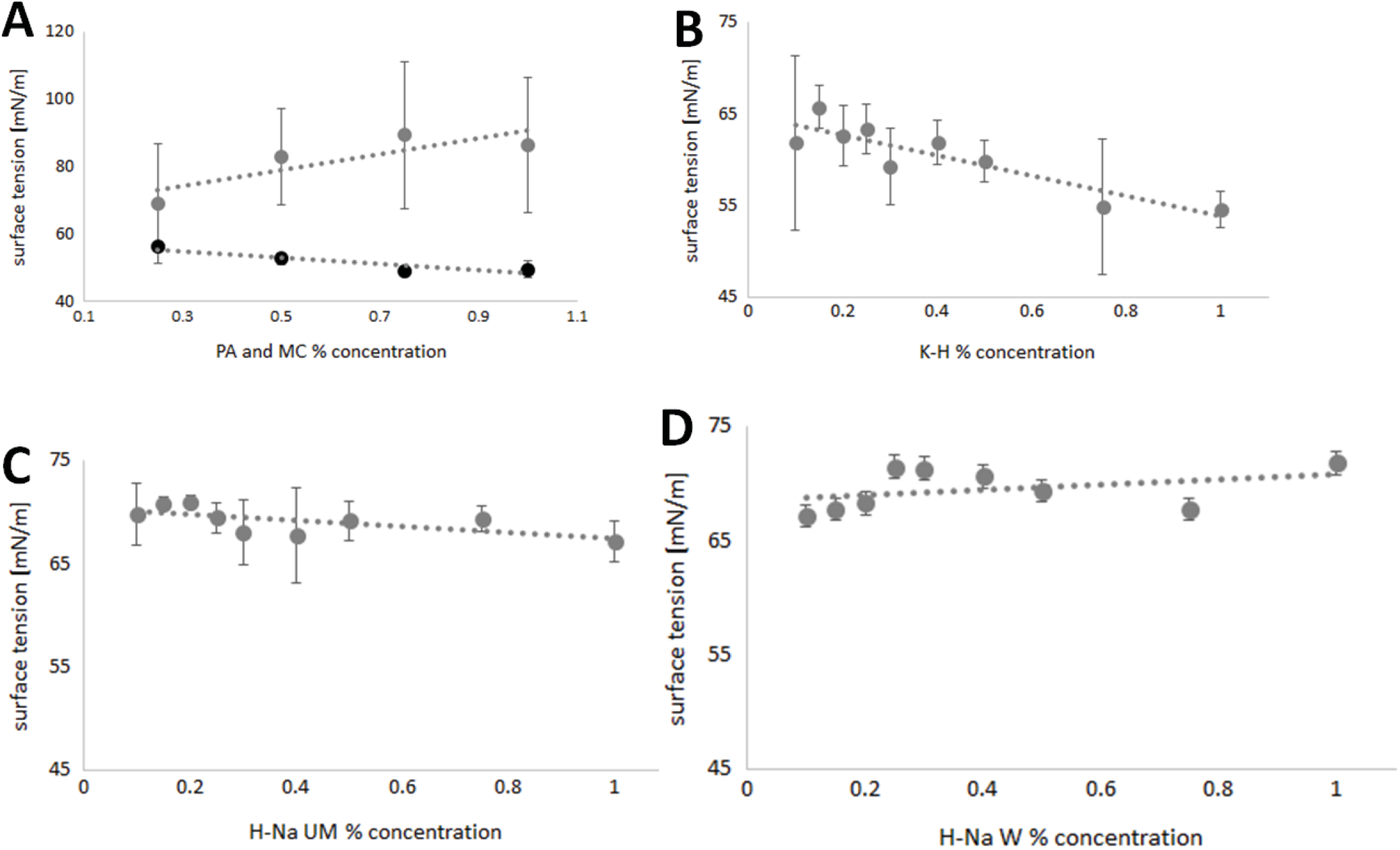
Results. The research presented in this paper shows the various effects of different eye drop ingredients on the surface tension of the solutions. The surface tension values of PA solutions are in the range of 48.89–56.03 mN/m, of MC in the range of 68.94–89.32 mN/m, of K-H 54.54–65.66 mN/m, of H-Na UM 67.18–70.97 mN/m, and of H-Na W 67.09–71.73 mN/m.
Conclusions. The use of different polymers affects the surface tension of model solutions proposed for use in ophthalmic preparations. Compounds containing carboxyl groups and anionic polymers have a similar effect on reducing the surface tension of the solution as classical surfactant compounds.
Key words: eye drops, surface tension, hyaluronic acid, polyacrylic acid, methylcellulose
Streszczenie
Wprowadzenie. Aby zwiększyć lepkość kropli do oczu, a także zmniejszyć ich napięcie powierzchniowe, stosuje się wiele substancji. Ich funkcją jest przedłużenie trwałości preparatu na powierzchni gałki ocznej oraz zwiększenie biodostępności substancji farmakologicznie czynnej.
Cel. Celem badań było zbadanie napięcia powierzchniowego substancji dodawanych do kropli do oczu, których głównym celem była modulacja właściwości preparatu.
Materiały i metody. Zbadano pięć substancji zawartych w roztworach proponowanych do opracowania kropli do oczu: hialuronian sodu wielkocząsteczkowy, hialuronian sodu ultramolekularny, kwas hialuronowy 4%, metylocelulozę i kwas poliakrylowy. Główną metodą było badanie napięcia powierzchniowego za pomocą tensjometru z wykorzystaniem pierścienia du Noüye.
Wyniki. Badania przedstawione w artykule pokazują zróżnicowany wpływ różnych substancji przeznaczonych do kropli do oczu na napięcie powierzchniowe roztworów. Wartości napięcia powierzchniowego roztworów PA mieszczą się w przedziale 48,89–56,03 mN/m, MC w przedziale 68,94–89,32 mN/m, K-H 54,54–65,66 mN/m, H-Na UM 67,18–70,97 mN/m, oraz dla H-Na W 67,09–71,73 mN/m.
Wnioski. Zastosowanie różnych polimerów wpływa na napięcie powierzchniowe modelowych roztworów do stosowania w okulistyce. Związki zawierające grupy karboksylowe – polimery anionowe, wpływają na obniżenie napięcia powierzchniowego roztworu podobnie jak klasyczne związki powierzchniowo czynne.
Słowa kluczowe: krople do oczu, napięcie powierzchniowe, kwas hialuronowy, kwas poliakrylowy, metyloceluloza
Background
Eye drops are the most popular method of administering ocular medications, not only for the treatment but also for the prevention and relief of symptoms of many diseases. They are a relatively safe way of delivering many groups of drugs, including antibiotics and anti-inflammatory drugs, to the ocular surface. During manufacture, a number of excipients are added to increase eye drops bioavailability, ensure optimal storage conditions, and prolong the action and persistence of the active ingredients at the site of application.1
To increase the bioavailability of eye drops, a number of substances are used to increase the viscosity of the drops at the site of application and to reduce their surface tension. Their function is to prolong the persistence of the product on the ocular surface.2
There are forces between the molecules of a liquid that determine its cohesion, i.e., surface tension forces. They are the result of asymmetric forces acting on the surface of liquids. In the depths of the liquid, the are no forces acting on the particles because all the particles surround each other and are in equilibrium. However, at the surface, at a thickness of about 10 nm, the forces are not balanced because there are not enough interactions from the free surface. The unbalanced force is called the surface tension or surface tension coefficient, and is defined as the force exerted by a single free surface of a liquid per unit length of its circumference. The value of the surface tension is expressed in [N/m] and [J/m2]. Surface tension measurements are mainly used to characterize solutions containing amphiphilic substances.3, 4
The reduction of surface tension values can be critical in assessing the quality and manufacture of many dosage forms, including emulsions, inhalation preparations, and eye drops. The substances that enable this phenomenon to occur are surfactants, tensides, and emulsifiers. They are characterized by an amphiphilic structure, which has both lipophilic and hydrophilic parts in its structure. This allows the surfactant molecules to properly align on the surface of the solution and form micelles, which affect the reduction of the surface activity value. Surfactants have the ability to reduce this value to a certain limit, which cannot be exceeded despite increasing the surfactant concentration. This limit is called the critical micellar concentration.5, 6
The addition of most compounds to a pure solution results in a reduction in surface tension. In particular, insoluble organic compounds have good surfactant properties due to their structure and high content of -CH2- groups. These include lipids, which are often used in the manufacture of medicines and in everyday life. Surfactants can be divided into cationic (containing an amine or ammonium group in their structure), anionic (with a carboxyl group), and non-ionic (made up of sugar molecules or oligooxyethylene groups). The substances most commonly used to reduce surface tension in the manufacture of eye drops are Tween 80 and some preservatives such as benzalkonium salts.6, 7
The study focused on investigating the surface tension of substances added to eye drops whose primary purpose is not to modulate the surface tension of the formulation. The aim of the study was to investigate the effect of the diversity of raw material composition of eye drops on their surface tension value.
Materials and methods
Sodium hyaluronate macromolecular (H-Na W) of molecular weight of 1.15 MDa (Escent, Szczecin, Poland), sodium hyaluronate ultramolecular (H-Na UM) of molecular weight of 4.5 kDa (Escent), hyaluronic acid 4% (K-H) (Escent), polyacrylic acid Carbopol®980 NF (PA) (Lubrizol, Wickliffe, USA), methylcellulose (MC) (Sigma-Aldrich, St. Louis, USA), and deionized water were obtained from the ionic column according to monography of the purified water from the European Pharmacopoeia; this type of water was used in all studies.
Composition of prepared solutions
Appropriate amounts of substances and deionized water were weighed out on an analytical balance. The solids of PA, MC, H-Na W, and H-Na UM were then combined with deionized water. The prepared solutions were allowed to dissolve completely at 8°C under a cover. Polyacrylic acid-based solutions were neutralized with an appropriate amount of NaOH. Previously prepared solutions of H-Na W and H-Na UM mixtures were used to prepare solutions of these substances. Ten milliliters of each solution was weighed on an analytical balance and mixed with deionized water in a ratio of 1:1. All solutions were stored under a cover at 8°C. The prepared solutions shown in Table 1.
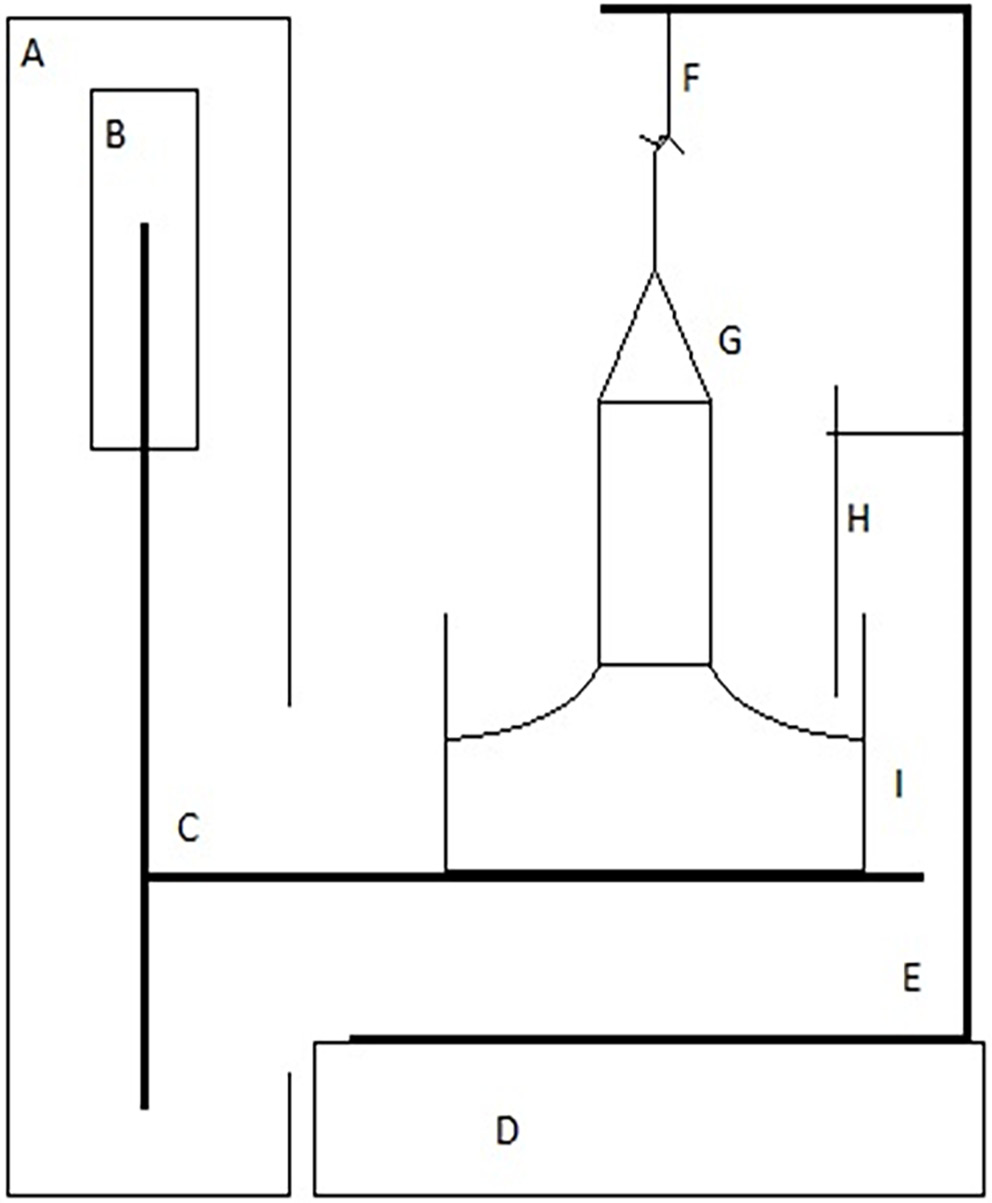
To measure the surface tension of prepared solutions, the formulations were placed in a dry measuring vessel, the du Noüy ring was immersed in the liquid, and the platform was slowly lowered. This method tested the force required to completely detach the ring from the surface of the solution. The schematic design of the tensiometer is shown in Figure 1. Individual results have been calculated directly using D-MT1A.exe software (Polon Izot, Warsaw, Poland)using the following formulas (Equation 1):
 (1)
(1)
S – surface tension in [mN/m];
Fmax – force [N];
K – calibration factor automatically determined by the program;
R – ring radius [mm];
f – correlation coefficient determined according to the Zuidema–Waters formula (Equation 2):

SPOM – surface tension value [mN/m];
R – radius of the ring [mm];
r – wire radius [mm];
Δρ – difference in density between the tested liquid and air [g/mm3].
Results
Changes in the surface tension values of different concentrations of PA are shown in Figure 2A. The highest surface tension was observed for the 0.25% solution. The decrease in surface tension is clearly visible in solutions with higher concentrations except for the 1.0% solution. The surface tension values are in the range of 48.89–56.03 mN/m. In Figure 2A, we can observe clear differences in the surface tension of MC solutions. The surface tensions of solutions with concentrations from 0.25% to 0.75% have an increasing trend, while the tension of the 1.00% solution is lower than the previous one by 2.96 mN/m. Figure 2B–D shows the surface tension values of K-H, H-Na UM, and H-Na W solutions. The surface tension values of K-H decrease with increasing concentration, oscillating in the range of 54.54–65.66 mN/m. In the H-NA UM solutions, the lowest surface tension values were recorded for concentrations of 0.30% and 0.40%. The minimum surface tension result was 67.18 mN/m and the maximum was 70.97 mN/m. Pointing out the surface tension of H-Na W, it can be observed that clearly the highest surface tension is shown by the 0.25% and 1.00% solutions: 71.43 mN/m and 71.73 mN/m, respectively. The smallest values are shown by the 0.10% solution: 67.09 mN/m.
Discussion
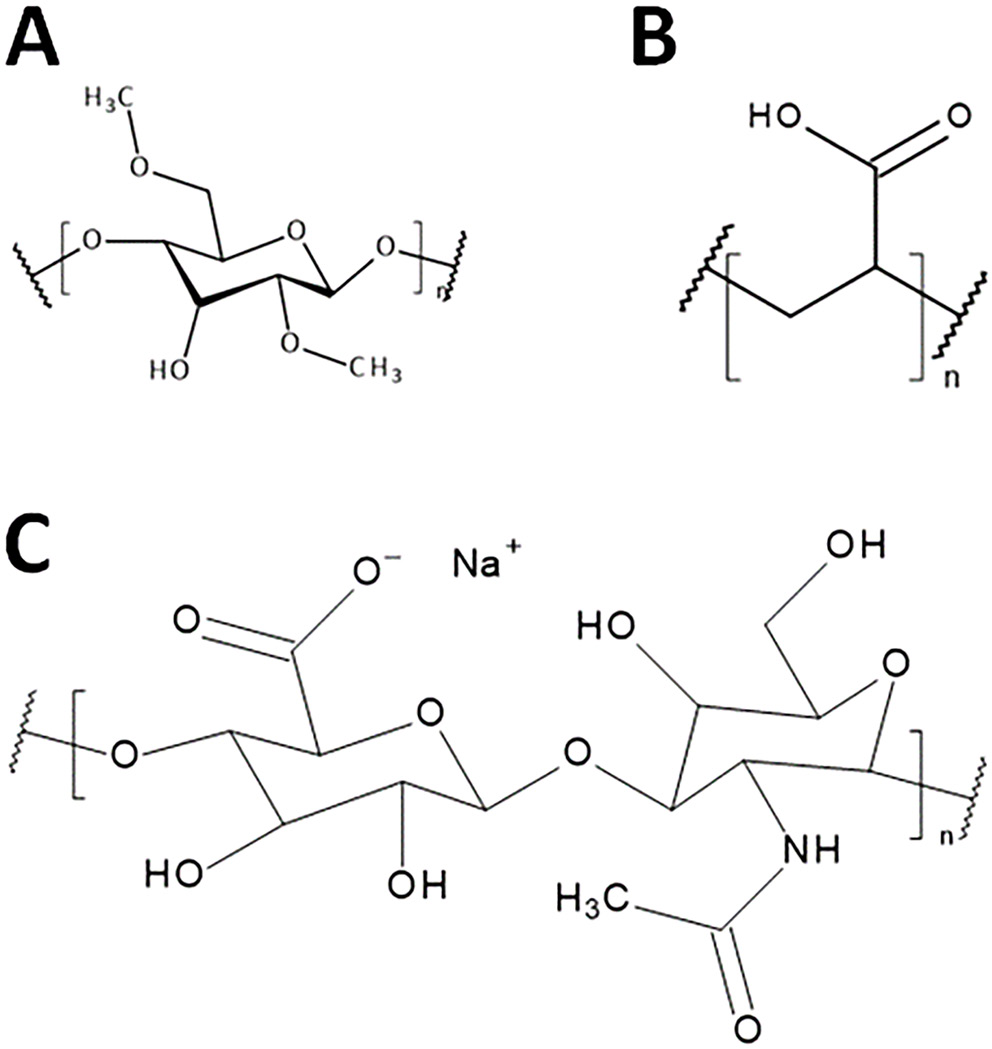
The research presented in this paper shows different effects of selected polymers on the surface tension of eye drop formulations. The experiments were carried out using deionized water and solutions of PA, MC, K-H, and their salts of different molecularities at different concentrations. The structural forms of the polymers used are shown in Figure 3. During the measurement of the surface tension with the tensiometric method using the du Noüy ring, the recorded values of the surface tension increased up to a certain maximum.8 Over time, they reached an extreme, after which they decreased, and the reading at the highest point was the correct value of this voltage. The shape of the curve obtained in the experiment results from the interactions between individual molecules of the substance. The strength of these interactions depends on many factors, including temperature and small differences in composition, such as the absorption of CO2 from the air by the solution. Intermolecular interactions in water solutions are mainly based on the forces of repulsion and attraction between molecules, the strongest of which are the forces between ions in electrolyte solutions and the van der Waals forces. It is these interactions, which can be perturbed by the phenomena of physical adsorption and chemical adsorption, that are important in surface tension measurements. Polymers such as PA and MC are used as thickeners and viscosity enhancers in ophthalmic products.9 The acrylic acid polymer, which contains free carboxyl groups, has a clear effect in reducing the surface tension of the system. In contrast, in the case of the non-ionic polymer shown in Figure 2, an increase in surface tension with increasing polymer concentration is observed over a relatively wide range of concentrations.
The presence of carboxyl and hydrophobic groups may promote a reduction in the surface tension of the PA solution in a manner analogous to that of surfactants. In the case of MC, the absence of ionized groups suggests that cohesion forces between linear polymer chains are responsible for the measured results. These forces increase with increasing concentration and the number of polymer chains present in the solution.10, 11 There are many scientific papers in which K-H molecules of different molecular weight and their salts have been characterized using traditional structural methods such as Fourier-transform infrared spectroscopy (FTIR), nuclear magnetic resonance (NMR), scanning electron microscopy (SEM), or X-ray diffraction.12, 13, 14 Surface tension is not a commonly studied parameter in solutions of K-H and its salts, but it is very useful in the treatment of certain diseases, especially those associated with dry eye syndrome.15 In the case of pure K-H, the surface tension values shown in Figure 2B tend to decrease with increasing concentration. It is likely that in this case the interactions of the polymer with the solvent took place on a similar basis as in the case of PA. Polymers containing carboxyl groups can be compared with surfactants containing the corresponding ionized group and aliphatic chain.16 Unfortunately, the sodium salt of K-H did not give clear results on the effect of its concentration in solution on the surface tension in the cited study. This knowledge was used to evaluate the interactions between the molecules. The results confirm that the addition of sodium ions is likely to have a major effect in stabilizing the surface tension. Compared to MC and PA, the system containing the sodium salt of K-H is slightly more complex. In this system, the surface tension is influenced not only by the polysaccharide chain with its carboxyl groups, but also by the sodium ions.17 The result obtained from the surface tension measurements may be a result of the influence of the above factors. This is confirmed by the fact that the surface tension values of H-Na UM and H-Na W are directly independent of the polymer concentration. In the case of a short chain polymer, the observed surface tension values initially tend to increase, after which the surface tension decreases and then increases again. The same is true for H-Na W, but probably due to interactions between the polymer chains, the values are slightly shifted.15 In the next stage of the planned research, it is advisable to carry out viscosity tests, as the surface tension of liquids depends on many factors, including viscosity.18 In 1966, Pelfosky presented the first possible relationships between the natural logarithm of surface tension values and the reciprocal of viscosity.19 Today, his research is constantly being refined and extended, allowing the introduction of more variables. Many scientists are also developing their own methods for studying these relationships, as evidenced by the publication of Vaziri et al.20 who presented 2 new methods for calculating surface tension values based on surface tension relationship with particle size, density, and viscosity of the solution. They showed that in the ionic solutions studied, the surface tension decreases as the length of the cationic alkyl chain increases. This is due to a decrease in van der Waals forces and the aliphatic nature of the solution as the length of the anion increases. This has the effect of reducing electrostatic forces in the solution and increasing dispersion,19 which confirms the importance of intermolecular forces, cohesion, and adhesion in the formation of surface tension.
Conclusions
1. Measurement conditions using the du Noüy ring have a significant effect on the recorded surface tension.
2. Higher surface tension values and higher standard deviations were observed for MC compared to PA.
3. Hyaluronic acid 4% surface tension values decreased significantly with increasing solution concentration, which was not observed for K-H salts.