Abstract
Background. Sida cordifolia and Sida rhombifolia are regarded as useful herbs as they have been shown to be effective, inexpensive and harmless in the prevention of diabetes, and are recognized as valuable therapeutic substances.
Objectives. The purpose of this study was to assess the effect of S. cordifolia and S. rhombifolia in the treatment of diabetic nephropathy using a rat model.
Materials and methods. Extracts of S. cordifolia and S. rhombifolia were obtained using the Soxhlet method. The hydroalcoholic extract solvent was used in the following proportions: 70:30, 50:50 and 80:20. The 80:20 hydroalcoholic extract was observed to be the most potent. The inhibitory effects of the extract were determined using the α-amylase assay. The most potent extract also underwent total flavonoid, phenolic and free radical scavenging tests, and was incorporated into an animal study. Diabetes was induced in rats by administering nicotinamide (NAD; 230 mg/kg) and streptozotocin (STZ; 65 mg/kg) intraperitoneally. In addition to a standard control of pioglitazone, the rats received extract dosages of 100 mg/kg/day or 200 mg/kg/day. Body weight, blood glucose, glycated hemoglobin (HbA1c), blood urea nitrogen (BUN), serum albumin, serum creatinine, homeostatic model assessment of insulin resistance (HOMA-IR), and oral glucose tolerance were assessed at various time points. The animals also underwent histopathological examination to observe alterations induced by the treatment.
Results. Sida cordifolia was the most successful in lowering blood glucose and HbA1c levels. Renal function indices and antioxidant enzyme levels were regained in a dose-dependent manner. Furthermore, S. cordifolia (200 mg/kg/day) extract, similar to pioglitazone, inhibited the production of advanced glycation byproducts by the kidney.
Conclusions. The effects of various S. cordifolia and S. rhombifolia extracts on rats with diabetic nephropathy were observed. Sida cordifolia may be further explored for the treatment of diabetic nephropathy and, due to its diverse nature, may be utilized for the treatment of a wide range of diseases, as it provided more significant findings.
Key words: Sida cordifolia, Sida rhombifolia, diabetic nephropathy, extraction, blood glucose, HbA1c
Background
The International Diabetes Federation (IDF) has reported the incidence of diabetes mellitus to be 8.8% among adults. Diabetes mellitus has impacted roughly 400,000,000 individuals, and the number is expected to rise to 550,000,000 individuals in the next decade.1, 2 One of the serious consequences of diabetes mellitus is diabetic nephropathy, also known as diabetic kidney disease. If not addressed, the disease causes from 20% to 40% of individuals to develop renal failure or blatant nephropathy due to untreated microalbuminuria within 2 decades. This condition is usually distinguished through early symptoms of renal cell death or failure, oxidative stress, expansion of interstitial fibrosis and mesangial matrices, bulking of basement membranes, and inflammatory responses.3 The activation of many biochemical and metabolic processes, including fluxes of glucose from polyol and hexosamine routes, along with the excessive or unnecessary activity of isoforms of protein kinase C and buildup of end products of advanced glycation, is caused by hyperglycemia. These pathways and routes are the primary cause of nephropathy and are therefore therapeutic targets in managing the disease.4
Herbs are considered valuable therapeutic substances, as they have been shown to be inexpensive, harmless and effective in the prevention of diabetes. Furthermore, using therapeutics derived from herbal plants is critical in underdeveloped nations due to the financial restraints related to conventional drugs. Therefore, there is an increased need to develop medicines that are cheap and efficacious.
Sida rhombifolia is a 1.5-meter tall upright shrub that is grown perennially or yearly and has stellate bristles and coarse stems. It belongs to the Malvaceae family, is also known as Atibala or Bala, and is among the 200 varieties of Sida. The plant has various therapeutic uses and pharmacological effects on cardiac, diabetic, rheumatic, and migraine-like issues, and is distinguished by its green diamond-structured leaf that has prickly stipules and grey hair-like features on the underside.5 Hypoglycemic and hypolipidemic effects of this herb have been observed in pre-clinical research on mice affected with diabetes. The effects of the plant are associated with its free radical scavenging activity, which has proven useful in indicating new roles for the herb.6
Similarly, Sida cordifolia, another potentially useful herb of the Malvaceae family, has proven to be an asset in traditional medicine. The herb is native to America and tends to grow in tropical areas in other parts of the globe, as it propagates fast in soils spoilt by grazing, heat and rain. Different parts of the herb are being used in day-to-day medicine for ailments related to respiratory and central nervous system, as well as inflammatory, tonic, diuretic, and various other disorders.7 Structurally, the herb is perennially upright and has a layer of white-colored hair that gives rise to its common name, “flannel weed”.8 In previous studies conducted on diabetic rats, reductions in blood glucose levels, triglycerides, plasma urea and creatinine, low-density lipids, and cholesterol were observed. Also, an increase in superoxide dismutase and catalase enzymes that are required for antioxidant synthesis was reported.9 It has also been observed that S. cordifolia has nephroprotective effects, which may be due to its antioxidant properties.10
Both plants have shown the potential to exhibit hypoglycemic effects that may help to reduce the damage caused by diabetic nephropathy. In the present study, extracts of S. cordifolia and S. rhombifolia were individually evaluated and compared in rats with induced diabetic nephropathy, through the observation of changes and protective effects.
Materials and methods
Experimental model
The study used adult male Wistar rats weighing 250–300 g. The rats were given a conventional rodent diet along with water, and were housed at a temperature of 23 ±2°C with a 12-hour day-night cycle.
Plant material collection
The aerial part of 2 species of the plant, S. cordifolia and S. rhombifolia, were obtained from Varuni Exports (Chennai, India).
Extraction
The aerial parts of S. cordifolia and S. rhombifolia were dried, crushed into powder form and kept in air-tight bags at approx. −80°C. The extraction was performed using the Soxhlet method, which employs water/ethanol (80:20) and distilled water as the solvent solutions. Constituents with antidiabetic effects were derived and added separately into solvents in hydroalcoholic solution. The procedure for extraction was repeated until the mixture showed no color, and the mixture was then distilled. Then, the mixture was later concentrated and freeze-dried under low pressure. Further extractions were conducted using hydroalcohol in varying ratios, including 70:30 and 50:50, to find the most potent extract. Such extract was observed during the procedure that used a ratio of 80:20.
α-amylase assay
Porcine pancreatic α-amylase (0.5 mg/mL) was prepared in an aqueous solution containing 1% starch and 20 mM sodium phosphate buffer. Stock solution A was prepared by adding 1 M sodium phosphate monobasic monohydrate (NaH2PO4.H2O, molecular weight: 138) and 2.76 g of NaH2PO4.H2O (monobasic) to a final volume of 1 L of water. Stock solution B was prepared by adding 1 M sodium phosphate dibasic (Na2HPO4, molecular weight: 142) and 2.84 g of Na2HPO4 (dibasic) to a final volume of 1 L of water. Stock solution A (450 mL) and 0.3504 mg sodium chloride (NaCl) were added to stock solution B (550 mL). The 2nd reagent, dinitrosalicylic acid (DNS) (molecular weight: 228.12), was prepared by warming and constantly stirring 20 mL of deionized water with 96 mM DNS, without boiling. The final reagent was prepared by dissolving 2 M sodium hydroxide (NaOH, molecular weight: 0.7999) in 8 mL of 5.31 M sodium potassium tartrate, and 10 mL of 2 M NaOH in water. This solution was warmed and stirred on a heating/stirring plate without boiling. Throughout the stirring, reagent A was gradually added to reagent B and diluted to 40 mL with deionized water. The colored reagent solution was then stored in an amber container at ambient temperature for up to 6 months.
Assay procedure
Multiple extract preparations were produced in a broad range of concentrations (0.1–1000 µg/mL). Extracts (100 µL) were then mixed with 100 µL of a 1% starch solution prepared in 20 mM trisodium phosphate (Na3PO4) buffer. This solution was transferred to microtubes and maintained at 25°C for 10 min, after which 100 µL of porcine α-amylase (0.5 mg/mL) was added to each tube. The preparations were then incubated for 10 min at 25°C. Next, 200 µL of DNS reagent was added to each tube. The tubes were incubated at 100°C for 5 min. This was performed to keep the samples cool. Each tube had 50 µL of material removed and transferred to 96-well microplates. To dissolve the solution in each well, water was added (200 µL). The absorbance of each well was read at 540 nm. In addition, blank measurements were recorded from wells that did not contain the enzyme, and the results were compared to the control, acarbose. Conventional procedures were used to assess α-amylase assay activity, as follows (Equation 1):
 (1)
(1)
Chemicals
The chemicals used in the study were of analytical grade. Nicotinamide (NAD) and streptozotocin (STZ) were acquired from Sigma-Aldrich (St. Louis, USA). The kits for diagnosing biochemical estimations were purchased from Krishgen BioSystems (Mumbai, India).
Phytochemical screening
Using previously established methods with some modifications, chemical compounds including amino acids, alkaloids, flavonoids, tannins, carbohydrates, fats, terpenoids, oils, phenols, saponins, glycosides, and proteins were measured in both plant extracts.11
Estimation of total flavonoid composition
Total flavonoid content was determined using an aluminum chloride colorimetric test. Reference solutions of quercetin at concentrations of 30, 40, 50, 60, 70, 80, 90, and 100 g/mL were prepared in 96% ethanol. Also, 150 mL of 10% aluminum chloride was combined with 10 mL of reference solution in 96% ethanol. A 10 mL volume of 1 M sodium acetate was then combined with the mixture in a 96-well plate. Ethanol was used as a blank reagent in 96% of the cases. The reagents were subsequently kept at room temperature for 40 min, sheltered from light. The VersaMax Microplate Reader (Merck Millipore, Burlington, USA) was used to measure the absorbance at 415 nm. The obtained composition was presented as milligrams of quercetin.
Estimation of total phenolic composition
The Folin–Ciocalteu technique was used to measure total phenolic content. Throughout a flat-bottom 96-well microplate, 25 mL of extracts diluted from each part of S. cordifolia and S. rhombifolia were mixed with 100 mL of 1:4-diluted Folin–Ciocalteu reagent and stirred for 1 min. After 240 s, a 75-mL solution of sodium carbonate (Na2CO3; 100 g/L) was combined and spun at a reasonable pace for 1 min. After 240 s, a 75-mL concentration of Na2CO3 (100 g/L) was combined and spun at a reasonable pace for 1 min. The VersaMax Microplate Reader (was used to measure the absorbance at 765 nm over 2 h at room temperature. The absorbance activity was calculated from ethanol absorbance activities. Calibration was performed using dilutions of a gallic acid standard (C7H6O5; 10–200 mg/L). Gallic acid equivalents (GAE) from the plant were used to determine the total phenolic content in milligrams per gram of extracts.
Antioxidant activity
The antioxidant activity of extracts was determined by measuring their scavenging capacity using 2,2-diphenyl-1-picrylhdrazyl (DPPH). Tests were carried out on 96-well plates, using a 20-L solution of extract stock in each well. The quantities used were 2000, 1500, 1000, 500, and 100 ppm in 180 L of DPPH solution. The absorbance was measured 30 min after incubation using a VersaMax Microplate Reader. Measurements were recorded in a dimly lit chamber at 517 nm. Methanol was used as a procedural control, while ascorbic acid was used as a positive control. All tests were performed 3 times. The sample concentration required to inhibit DPPH by 50% was calculated (IC50 value). The percentage of scavenging capability was calculated using the following equation (Equation 2):
 (2)
(2)
Animals and design of experiment
To induce diabetic nephropathy, animals were injected intraperitoneally with NAD (230 mg/kg). After 15 min, STZ was dissolved in citrate buffer (pH: 4.5) and immediately injected intraperitoneally at a dosage of 65 mg/kg. Fasting blood glucose levels were measured 72 h after STZ injection to confirm diabetes. Only rats with fasting blood glucose levels ≥250 mg/dL were used in the study. After a literature search on acute toxicity studies, 2 dosages of extracts were chosen, 100 mg/kg and 200 mg/kg. All animal experiments were conducted following national guidelines and relevant national laws on the protection of animals. The experimental protocol (No. 1834) was approved by the Ethics Committee of Jamia Hamdard University (New Delhi, India; approval No. 1834).
Body weight and estimation
of blood glucose
Before STZ induction, body weight of each animal was measured and rats of comparable weight were housed together. Each group’s body weight was calculated and monitored regularly until the completion of the study. To confirm diabetes, glucose levels were measured 72 h after NAD/STZ injection. The fasting blood glucose level was measured at 15-day intervals using commercially available enzymatic kits (Krishgen BioSystems).
Enzyme-linked immunosorbent assay
for the assessment of blood parameters
A standard curve was constructed using buffer diluents covering a broad range of concentrations between 0 pg/mL to 3 times the highest predicted concentration of the antigen. The capture antibody was diluted to 15 μg/mL to a volume of 100 μL/well and incubated at 37°C for 2 h in a 96-well polystyrene plate, along with 100 µL of each standard. The solution from all of the individual wells was removed and replaced with a wash buffer (200 µL/well). The plate was then shaken for 5 min, and 200 µL of blocking buffer was added to each well. Next, the plate was covered and incubated at 37°C for 1 h. The wash buffer was removed, and the samples and standards were placed in separate wells and incubated at 37°C for 1 h. The 96-well plate was washed again. Detection antibodies were added to each well of the 96-well plate and incubated for 1 h before being washed again. The enzyme-conjugated secondary antibody (100 µL) was added to each well and the plate was incubated at 37°C for 1 h. The 96-well plate was washed again, twice, to minimize nonspecific binding. Horseradish peroxidase (HRP) substrate solution was added to each well (100 µL) and the plate was incubated at 37°C for 10 min until the color changed to blue. To turn the solution to yellow, 100 µL of stop buffer was added to each well, and the absorbance was measured at 450 nm using a plate reader (ELX800MS ELISA Reader; Shimadzu, Tokyo, Japan).
Tests for renal function
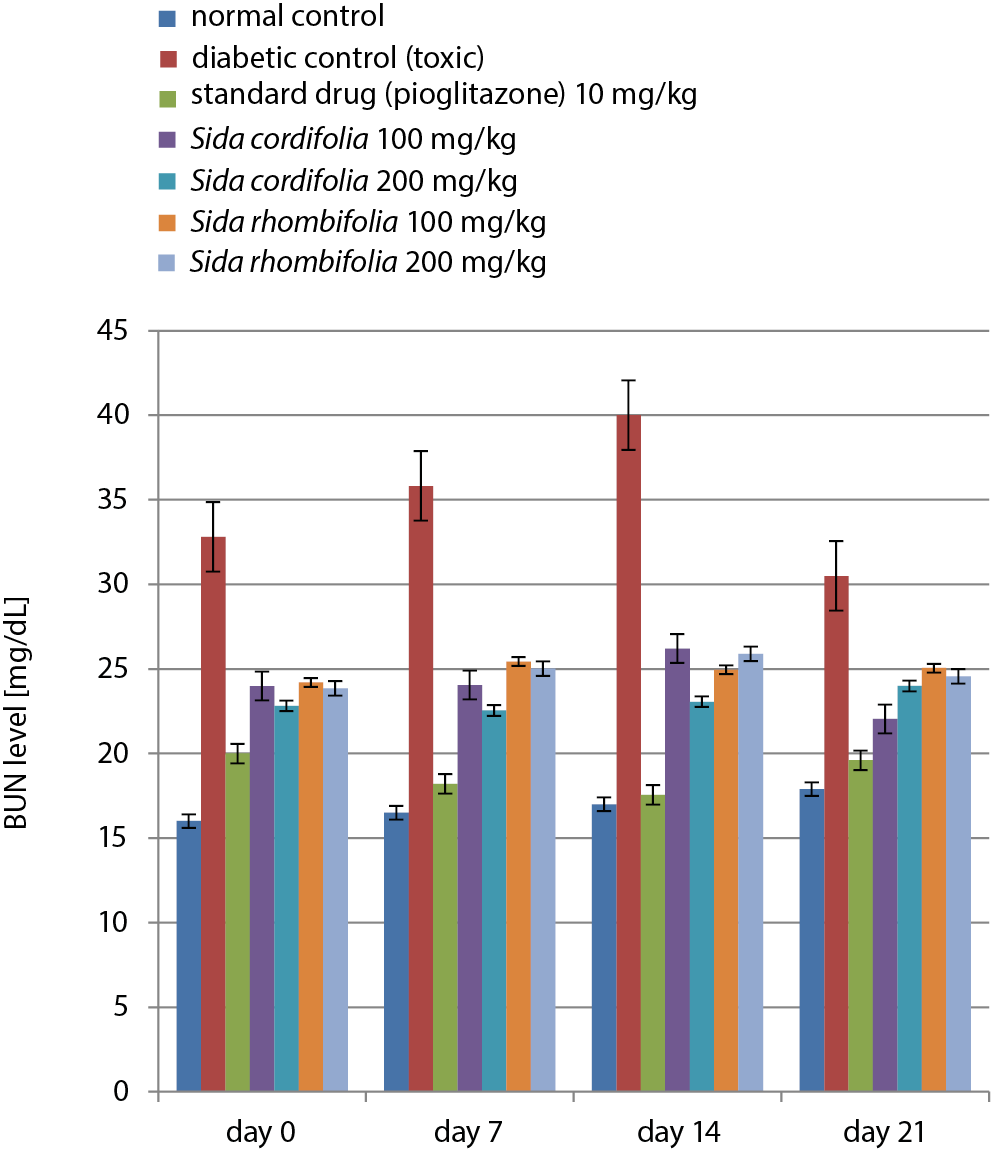
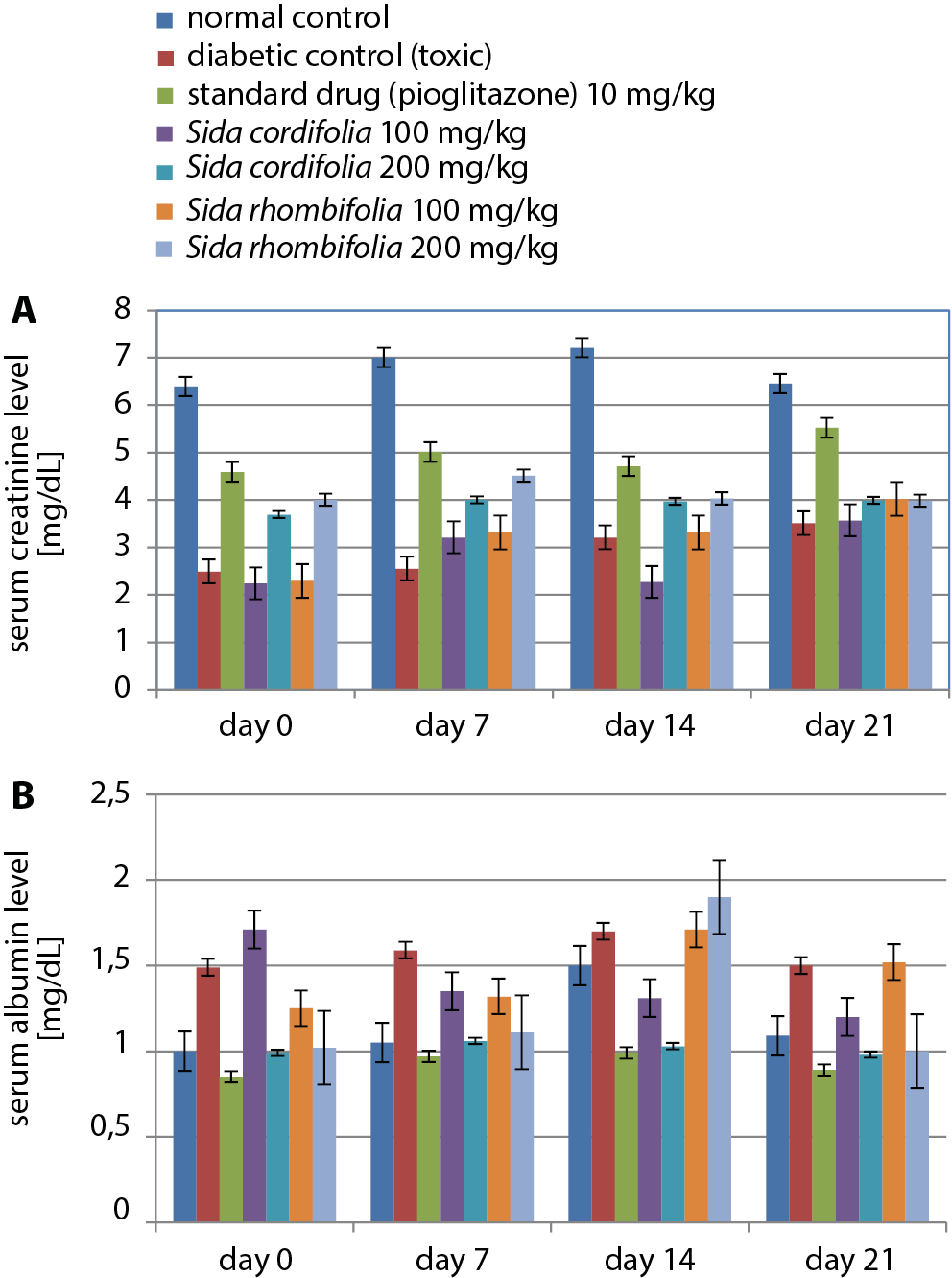
Samples of blood were analyzed using enzymatic kits (Krishgen BioSystems) to assess the control of diabetic nephropathy in rats that were treated for 0, 7, 14, or 21 days. This included estimates of blood urea nitrogen (BUN), serum albumin and serum creatinine.
Histopathology
Tissues were harvested from the pancreas and kidneys of euthanized animals and then dehydrated in ethanol, fixed in neutral buffered formalin solution (10%) and embedded in paraffin. To conduct a microscopic examination, 5-millimeter thick sections of tissue were collected using a rotary microtome LMIUK histopathology microscope (Sigma Histology,Center; New Delhi, India) and stained using hematoxylin and eosin (H&E).
Statistical analyses
GraphPad Prism v. 6 (GraphPad Software, San Diego, USA) was utilized for the analysis of data. The values from statistical analysis are presented as mean ± standard error of the mean (M ±SEM). Data were analyzed using one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test for multiple comparisons. The value of p ≤ 0.05 was considered statistically significant.
Results
α-amylase assay
Extracts with effective α-amylase inhibition were discovered. The hydroalcoholic extract with an IC50 concentration of 70.82 ±0.28 mg/mL was determined to be the most effective for further examination. Table 1 summarizes the extraction profile used to find the most effective fraction.
Total flavonoid composition
The potency of the hydroalcoholic extracts derived from the extraction tests was assessed. The total flavonoid content was expressed in terms of the milligram of quercetin equivalent per gram of the dry mass (mg QE/gm). The quercetin standard calibration curve for total flavonoid concentration in both plant extracts is reported in Table 2 as Y = 0.0613x + 0.0004, R2 = 0.9872. Sida rhombifolia had a greater total flavonoid concentration, 35.12 ±0.49 mg QE/gm, than the other plant extract.
Total phenolic composition
The experiments yielded potent hydroalcoholic extracts. Table 2 shows the total phenolic content of the aerial parts of both plants, together with a calibration curve (Y = 0.0371x + 0.0234, R2 = 0.9930). Sida rhombifolia produced a greater total phenolic content than the other plant, with 5.395 ±0.81 mg GAE/gm.
Antioxidant scavenging activity
The potent hydroalcoholic extract was used to evaluate the antioxidant scavenging activity. Table 2 shows the antioxidant scavenging activity of aerial sections of both plants, with S. rhombifolia exhibiting a greater scavenging activity than the other plant, with 92.13 ±2.4 IC50μg/mL of free radicals.
The extracts were used on the animals and the parameters described in the following sections were analyzed.
Effects of Sida cordifolia and Sida rhombifolia extracts on body weight
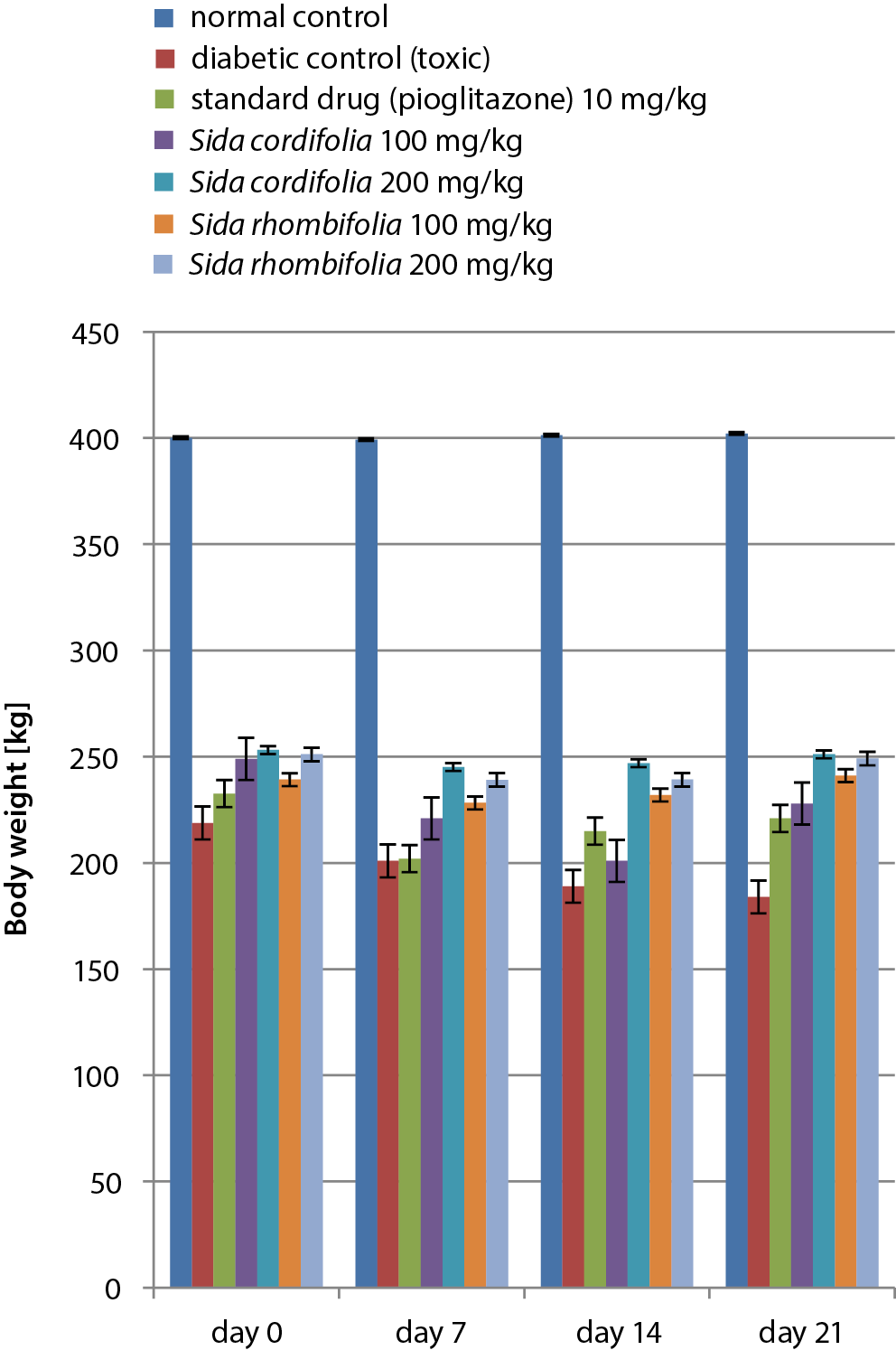
Substantial weight loss was observed in the experimental animals. The doses of extracts administered were 100 mg/kg and 200 mg/kg, with body weight reductions occurring in a dose-dependent manner. Pioglitazone also decreased the body weight of rats affected by diabetic nephropathy. The results of the body weight tests conducted are presented in Figure 1 and Table 3.
Effects of Sida cordifolia and Sida rhombifolia extracts on blood glucose
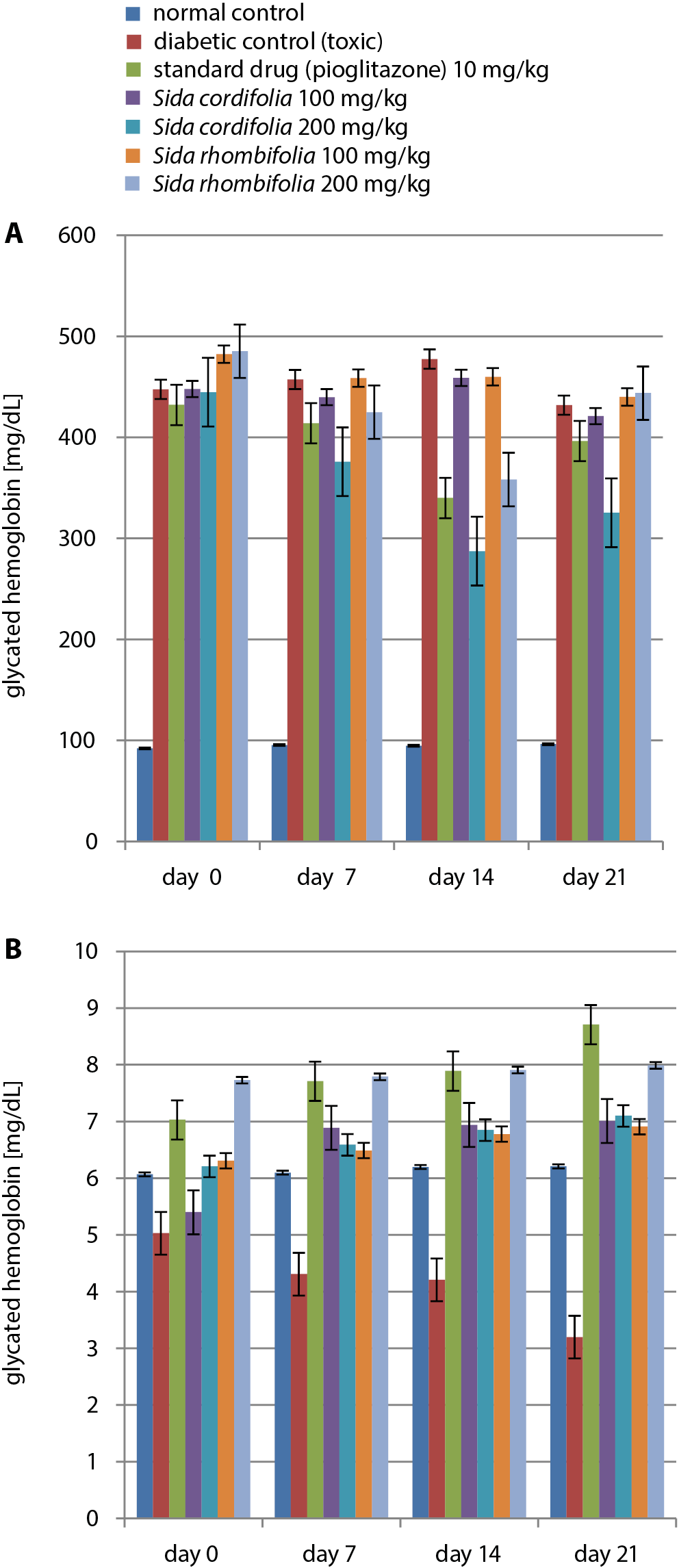
Various extract dosages were administered to rats and maintained for up to 21 days. Tests for the detection of blood glucose were conducted on days 0, 7, 14, and 21. During the 21 days, the fasting blood glucose levels varied from 92.1 mg/dL to 96.3 mg/dL in the control rats. At the same time, glucose levels observed in rats affected by diabetes were elevated to 431.8–477.4 mg/dL. Dosages of both extracts (100 mg/kg and 200 mg/kg) were administered for 21 days and resulted in a considerable modulation of blood glucose levels. The 200 mg/kg dosage of S. cordifolia caused the greatest attenuation (287.3 mg/dL) when compared to the rats affected by diabetes. Pioglitazone therapy also led to a considerable reduction in the levels of blood glucose (339.84 mg/dL). The results of the blood glucose level tests are presented in Figure 2A and Table 4.
Effects of Sida cordifolia and Sida rhombifolia extracts on glycated hemoglobin
The assessment of glycated hemoglobin (HbA1c) levels was performed on days 0, 7, 14, and 21 of the experiment. A significant reduction in HbA1c levels was observed within the nephropathy control group (3.20 ±0.02 mmol/mol) when compared to the rats in the normal control group (6.21 ±0.05 mmol/mol) at the end of the study. The dosages of 100 mg/kg and 200 mg/kg of both extracts resulted in substantial reductions in HbA1c levels. For the S. cordifolia 100 mg/kg group, a value of 5.40 mmol/mol was recorded on day 0, while the highest value (7.99 mmol/mol) was observed in the S. rhombifolia-treated group (concentration: 200 mg/kg) on day 21. It was observed that 200 mg/kg of S. cordifolia caused a consistent rise from day 0 to day 21. The results of the HbA1c level tests are presented in Figure 2B and Table 4.
Effects of Sida cordifolia and Sida rhombifolia extracts on the homeostatic model assessment of insulin resistance
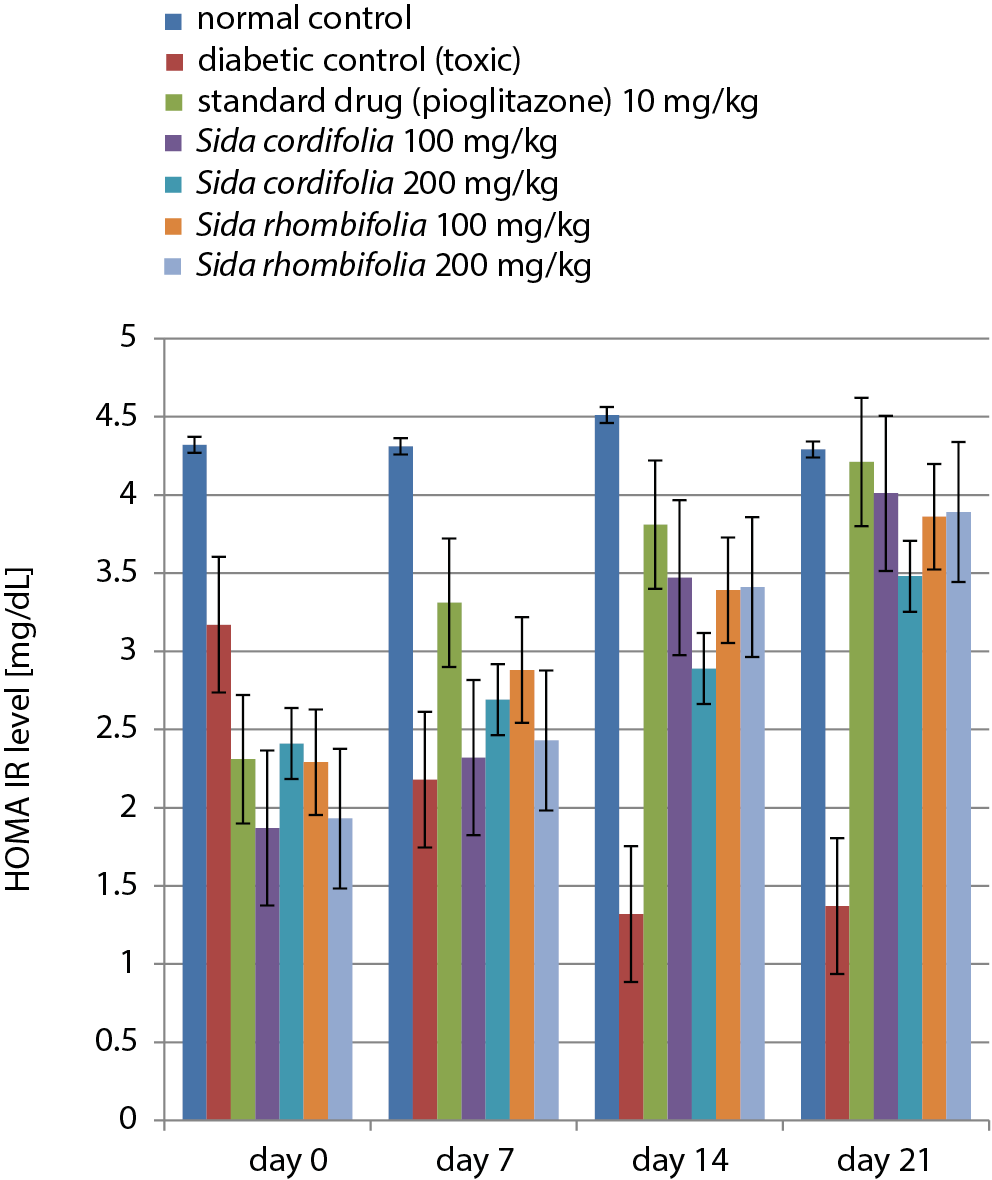
A dose-dependent increase was observed in the results of the homeostatic model assessment of insulin resistance (HOMA-IR) for all extracts and pioglitazone in the diabetic control group compared to the normal control group. The results of the HOMA-IR tests are presented in Figure 3 and Table 5.
Oral glucose tolerance test
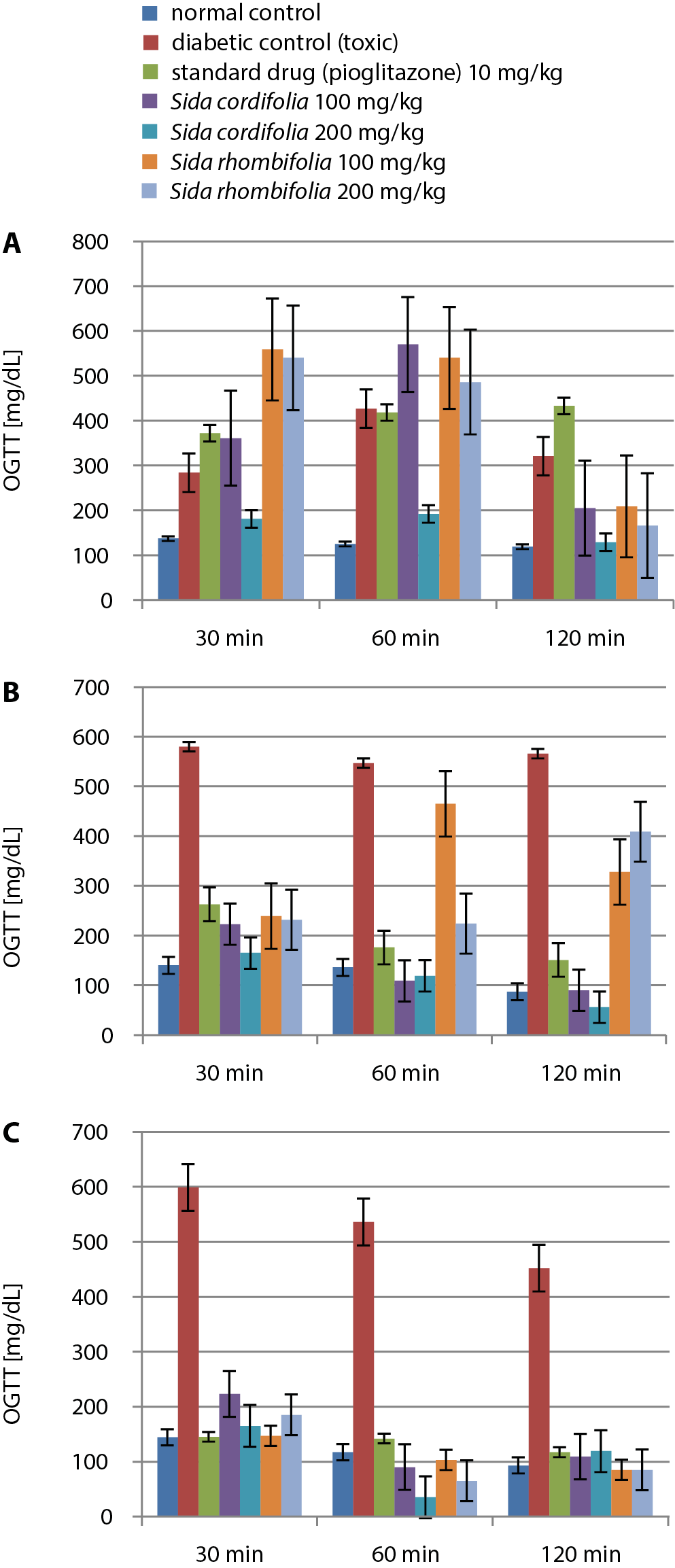
After conducting the oral glucose tolerance tests (OGTT) on day 7, we observed a similar reduction of glucose levels in the S.cordifolia (200 mg/kg) administered group along with the pioglitazone administered group at different time points of 30 min, 60 min and 120 min. Similarly, the group treated with S. cordifolia (200 mg/kg) had significantly decreased levels of glucose in the same timeframe. Other extracts caused increased oral glucose tolerance at a dosage of 200 mg/kg, except for S. rhombifolia, which considerably reduced the oral glucose tolerance in rats.
On day 14, it was observed that oral glucose tolerance had significantly decreased within 120 min in the normal and S. cordifolia 200 mg/kg groups. On day 21, it was observed that S. cordifolia (200 mg/kg) caused the lowest value for the OGTT at 60 min, which then rose by 120 min. The other doses of extracts caused a similar decrease and rise from 60 min to 120 min, while pioglitazone caused a considerable decrease throughout the discussed timeframe. The results of the OGTT tests are presented in Figure 4 and Table 6.
Effects of Sida cordifolia and Sida rhombifolia extracts on renal function
The study measured levels of BUN, serum albumin and serum creatinine.
Effects of Sida cordifolia and Sida rhombifolia extracts on blood urea nitrogen
During the 21 days of treatment, the BUN level of the normal control group was in a range of 16.01–17.9 mg/dL, while the diabetic control group had very high levels of BUN (40.01 mg/dL). Pioglitazone caused a considerable increase in BUN levels, while the extracts caused higher values of BUN. The results of the BUN level tests are presented in Figure 5 and Table 7.
Effects of Sida cordifolia and Sida rhombifolia extracts on serum albumin
During the 21-day treatment period, the normal control group had the highest serum albumin levels on day 14 (7.21 g/dL). Sida cordifolia (100 mg/kg) caused significantly decreased levels of serum albumin (2.24 ±0.45 g/dL), similar to those found in the diabetic control (2.49 ±0.19 g/dL) and S. rhombifolia-treated (100 mg/kg) animals (2.29 ±0.51 g/dL). Sida cordifolia (200 mg/kg), along with pioglitazone, caused a consistent change throughout the treatment period. The results of the serum albumin level tests are presented in Figure 6A and Table 8.
Effects of Sida cordifolia and Sida rhombifolia extracts on serum creatinine
Treatment with both extract dosages (100 mg/kg and 200 mg/kg) did not result in a considerable change in the levels of serum creatinine, although pioglitazone and S. cordifolia (200 mg/kg) had similar effects on this parameter. The results of the serum creatinine level tests are presented in Figure 6B and Table 8.
Histopathology of kidney tissues
from different groups
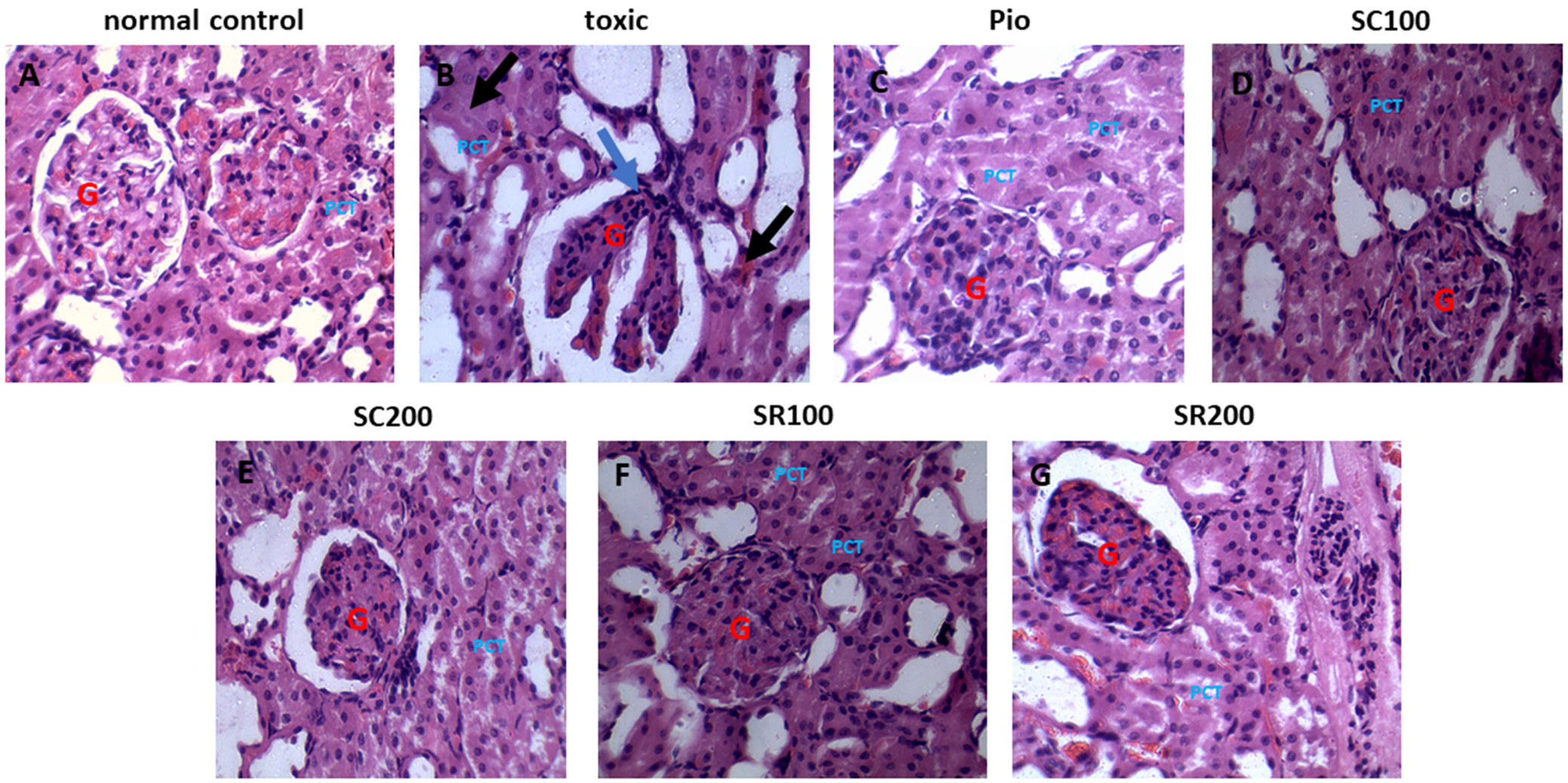
The rats in the normal control group exhibited normal Bowman’s capsules and parenchyma, with renal glomeruli enclosed by the distal and proximal tubules. The rats with STZ-induced diabetic nephropathy had severe congestion and hemorrhage of glomerular tufts, cloudy and swollen renal tubules, lymphocytic cell infiltration around glomeruli, and renal tubules with edema in the interstitial tissue. The pioglitazone-treated group had almost normal glomeruli and renal tubules. Sida cordifolia- (100 mg/kg and 200 mg/kg) and S. rhombifolia- (100 mg/kg and 200 mg/kg) treated animals showed less congestion and hemorrhage of glomerular tufts and some mildly swollen tubules. The S. cordifolia-treated group (200 mg/kg) also demonstrated protection against diabetic nephropathy, which was indicated by a decrease in atrophy, thickness of membranes and mesangial enlargements. Before the onset of microalbuminuria, oxidative stress in combination with persistent hyperglycemia may play an essential role in the etiology of tubular, glomerular, structural, and functional anomalies. Figure 7 depicts the histopathological changes observed in the kidneys of different groups.
Histopathology of the pancreatic tissue of control and treated animals
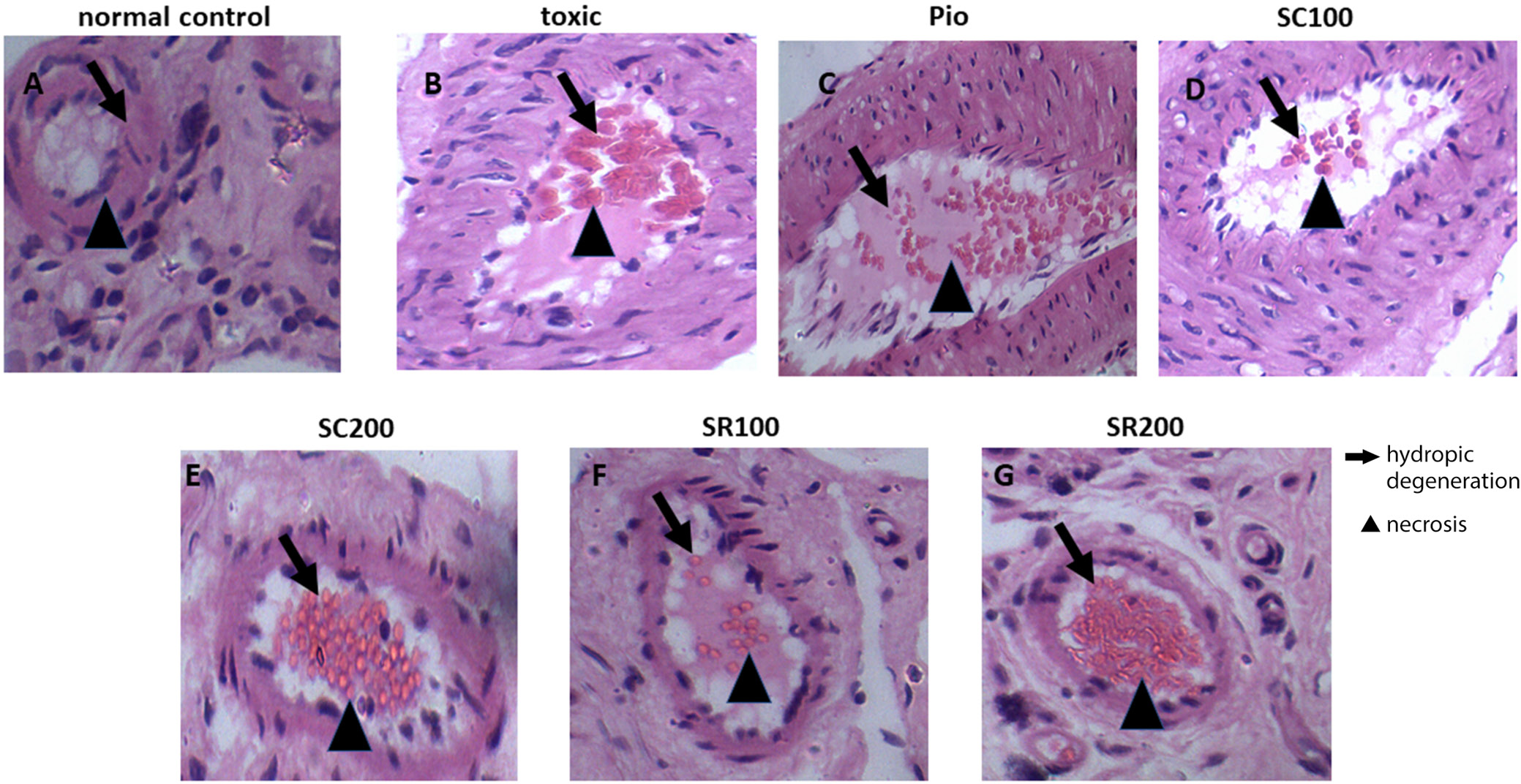
The normal control group was vehicle-treated and had a normal histological structure. The diabetic control group revealed atrophy in the islet of Langerhans, as well as degeneration and necrosis of islet cells. The group treated with pioglitazone (10 mg/kg/day) and S. cordifolia (200 mg/kg/day) had normal structures. The group treated with S. cordifolia (100 mg/kg/day) displayed atrophy, degeneration and necrosis in the islet of Langerhans. Sida rhombifolia treatment (100 mg/kg/day) resulted in a very mild atrophy in the islet Langerhans, along with the degeneration of islet cells and a little necrosis. Sida rhombifolia-treated rats (200 mg/kg/day) had a normal histological structure, although degeneration in islet cells and a little necrosis were observed. Figure 8 depicts the histopathological changes observed in the pancreatic tissue of the control and treatment groups.
Discussion
This study was conducted to examine the pharmacological activity of pioglitazone and extracts of S. cordifolia and S. rhombifolia in the treatment of rats with STZ-induced nephropathy. Blood glucose levels of the animals with STZ-induced nephropathy increased over the course of the experiment, which was significantly improved by a range of treatment dosages. Parameters, namely BUN, serum albumin and serum creatinine, were used to assess renal function and to diagnose nephropathy in diabetic rats. It was observed that among all of the extracts, S. cordifolia (200 mg/kg/day) improved kidney parameters and antioxidant levels. The antioxidant utilized in this study was NAD, which protects from the cytotoxic effects of STZ by eliminating free radicals. It achieves this while causing only modest harm to the β-cells of the pancreas. A similar situation occurs in type II diabetes. Nicotinamide, along with STZ, provides an excellent model for the study of pathophysiology of diabetes and is comparable to the human condition.12
The formation of chronic microalbuminuria is the initial clinical sign of emerging nephropathy in diabetes. If patients suffering from diabetes do not receive therapy or medication, around 20–40% of them will advance to extreme albuminuria, and another 20% will develop end-stage renal disease (ESRD) within the following 20 years. The pathogenesis of microalbuminuria is heavily influenced by oxidative stress and dysfunctional endothelial cells.13 In a previous study on type II diabetes conducted for over a decade, 38% of participants developed microalbuminuria that was indicative of nephropathy.14 In the current study, microalbuminuria was prevalent in the group of untreated diabetic rats, which was changed through the administration of plant extracts. High concentrations of metabolic waste products such as BUN, serum creatinine and serum albumin compromise the function of the renal system. The administration of different dosages of extracts led to considerable reduction in these parameters, and it was observed that the administration of S. cordifolia (200 mg/kg/day) resulted in the most effective response.
The HbA1c is an effective indicator for the management of hyperglycemia, as it represents the true levels of glucose in the blood, does not disintegrate quickly and is generated gradually. Furthermore, HbA1c levels have been reported to rise in diabetic individuals.15, 16 The administration of either S. cordifolia or S. rhombifolia decreased HbA1c levels in the blood and alleviated the hyperglycemic effects. The body weight of the treated animals was also lower. The most effective results, in comparison to pioglitazone, were achieved with S. cordifolia at 200 mg/kg/day.
Kidneys play a major role in eliminating and metabolizing serum, which increases during chronic insufficiency of the renal system. The STZ-induced rats had observable changes in renal functioning, similar to those seen in the nephropathy of diabetic humans. This included activation of nuclear factor kappa-B (NF-κB), expansion of mesangial matrices, overexpression of connective tissue growth factor (CTGF), hypertrophy of glomeruli, and thickening of glomeruli basement membranes.17 The histopathology reports for the group treated with S. cordifolia (200 mg/kg/day) were similar to the results obtained after pioglitazone treatment, and proved to be significant. These results can be utilized for the treatment of diabetic nephropathy and can stimulate further research into the use of the herb. However, it was observed that other doses of S. rhombifolia extract caused abnormalities in the kidneys and pancreas, making them unsuitable for further analysis.
Previous research findings suggest that S. cordifolia has the potential to exhibit nephroprotective properties. This could be due to the reduction of glucose pathways or through antioxidant activity.9, 18, 19 The plant is considered to be safe, with its median lethal dose (LD50) being greater than 3 g/kg. It may be of benefit in therapeutic practice as its toxicity potential is low.20 Our results suggest that further research is warranted on S. cordifolia (200 mg/kg/day) for the assessment and treatment of diabetic nephropathy.
Conclusions
In this study, the effects of S. cordifolia and S. rhombifolia extracts on rats affected by diabetic nephropathy were observed. The study highlighted and provided evidence that S. cordifolia (200 mg/kg/day) produced the most vital results by reducing HbA1c and blood glucose levels, along with having a significant impact on the results of the OGTT. Of the 2 plants, given their varied nature in the treatment of different ailments, S. cordifolia can be further studied and incorporated into the treatment of diabetic nephropathy.