Abstract
The introduction of tablet dosage forms has brought a revolution in the pharmaceutical drug delivery system. Different forms of tablets have been developed based on the target site, the onset of action, and therapeutic drug delivery methods. Fast-disintegrating tablets (FDTs) are the most promising pharmaceutical dosage form, especially for pediatric and geriatric patients having difficulty swallowing. The key feature of FDTs is quick drug release soon after their administration through the oral cavity. With innovations in the formulation of FDTs, the demand for excipients with better functionalities, particularly in terms of flow and compression characteristics, has increased. Co-processed excipients are a mixture of 2 or more conventional excipients that provides significant benefits over the individual excipients while minimizing their shortcomings. Such multifunctional co-processed excipients minimize the number of excipients that are to be incorporated into tablets during the manufacturing process. The present review discusses FTDs formulated from co-processed excipients, their manufacturing techniques, and the latest research, patents and commercially available co-processed FDTs.
Key words: flowability, fast disintegrating tablet, co-processed excipient, compressibility
Introduction
– fast-disintegrating tablets
Tablets are a widely accepted oral solid pharmaceutical dosage form around the world.1 Among these dosage forms, fast-disintegrating tablets (FDTs) have gained interest due to their rapid disintegration time.2 They were first developed in the late 1970s and have been of key interest to the pharmaceutical industry because of their enhanced bioavailability and rapid onset of action.3, 4 Dysphagia is the medical term for swallowing difficulties and is most frequent in geriatric and pediatric patients. To overcome such complications, FDTs are designed to exhibit quick breakdown within the oral cavity, hence eliminating the need for chewing and conjoint water consumption.5 They are also known as fast dispersing, rapidly dissolving, rapidly melting, and quick disintegrating tablets. Some commonly used disintegrants for the preparation of FDTs are starch, modified starch, sucrose, mannitol, microcrystalline cellulose (MCC), alginic acid, cross-linked polyvinyl pyrrolidone (PVP), and many more.6 As per the Food and Drug Administration (FDA), all FDTs are categorized under the Oral Disintegrating Tablets category.7, 8, 9 Orodispersible tablets are those that disperse in less than 3 min in the buccal cavity before swallowing. In the oral cavity, such tablets disintegrate into small granules or melt from a hard solid configuration into a gel-like structure that allows effortless swallowing of a drug. These tablets form a soft paste or liquid suspension in the oral cavity, providing a pleasant mouthfeel and effortless swallowing. Following their disintegration, there is minimal or no residue in the oral cavity.8, 9, 10
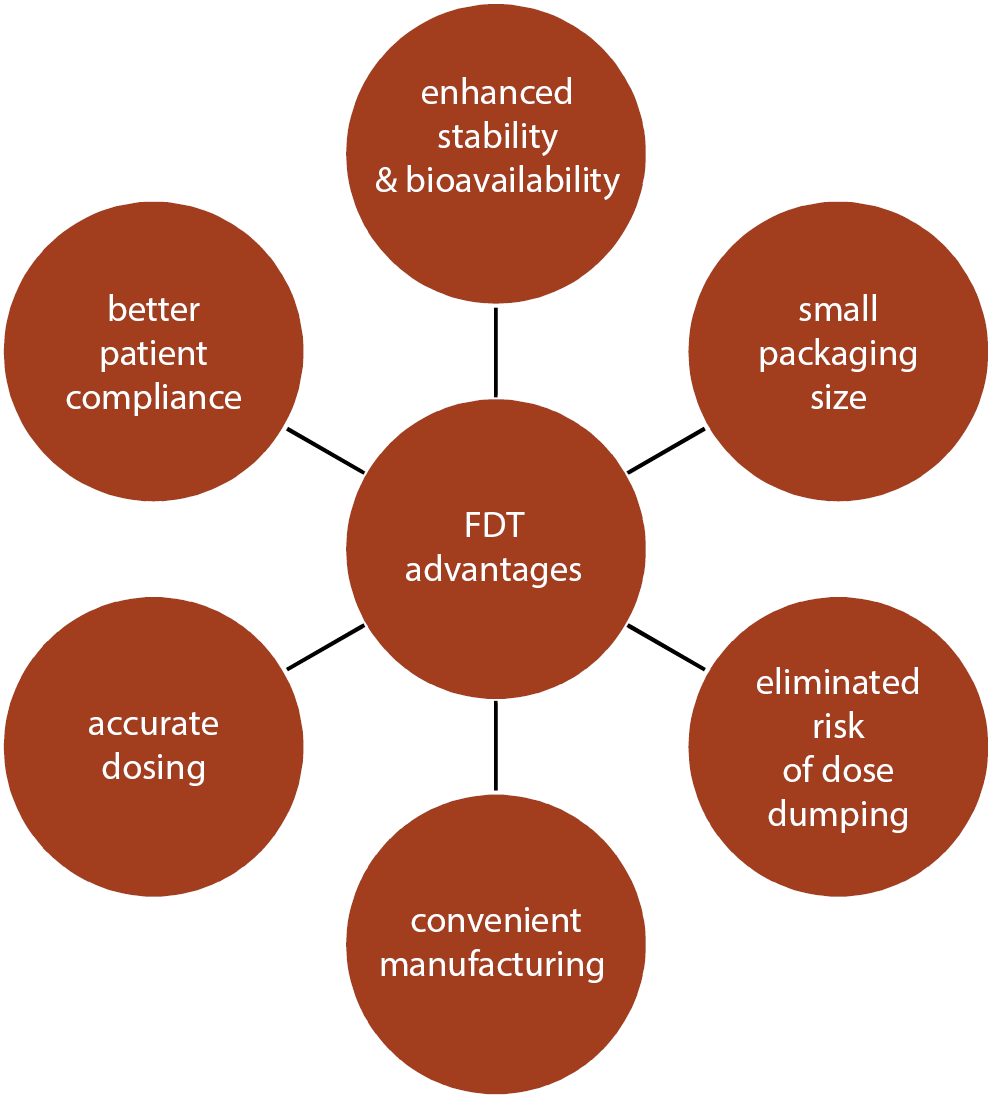
The FDTs have uniform advantages such as exceptional stability, ease of manufacturing and handling, good patient compliance, enhanced bioavailability and palatability, and accurate dosing.11 They are susceptible to humidity and temperature, and are suitable for patients that suffer from dry mouth and are on anticholinergic therapy. Moreover, FDTs disintegrate quickly and exhibit speedy absorption in the oral cavity. Such tablets lead to an increase in drug bioavailability, avoid first-pass metabolism and result in reduced dosing.9, 12 Some of the key advantages of FDTs are depicted in Figure 1.
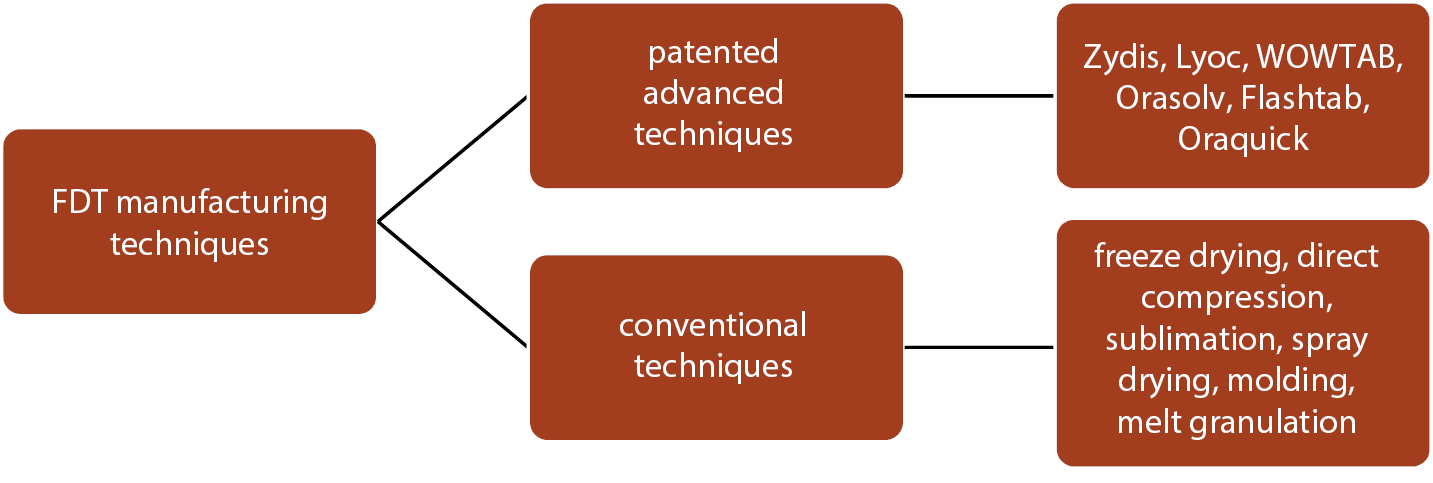
There are different patented and conventional manufacturing techniques for the preparation and development of FDTS, and several of them are listed in Figure 2.13 Table 1 summarizes the patented techniques used for the preparation of FDTs alongside their active ingredients and patent owner details.13, 14, 15, 16, 17, 18
Excipients
Excipients are non-therapeutic components that are part of any pharmaceutical formulation. These substances act as bulking agents and stability enhancers, and support the therapeutic efficacy of the active pharmaceutical ingredient.19, 20, 21 The most commonly used excipients in the tablet dosage form are diluents, binders, disintegrants, glidants, lubricants, surfactants, pH-adjusting agents, sugar (as sweetening agent), mucoadhesive polymers, and coating and coloring agents.22 Table 2 briefly presents the functionality of each excipient with examples.23, 24, 25, 26, 27, 28, 29, 30
For the development of FDTs, superdisintegrants are widely used in the pharmaceutical industry to minimize the disintegration time. Agar powder and amino acids are examples of naturally available disintegrants that are used in the preparation of FDTs.31Agar powder, due to its large porous size and overall volume, promotes rapid water permeation into the tablet, consequently resulting in fast tablet disintegration.32 Incorporation of amino acids such as proline and serine promotes rapid tablet disintegration due to enhanced wettability.33
Types of pharmaceutical excipients
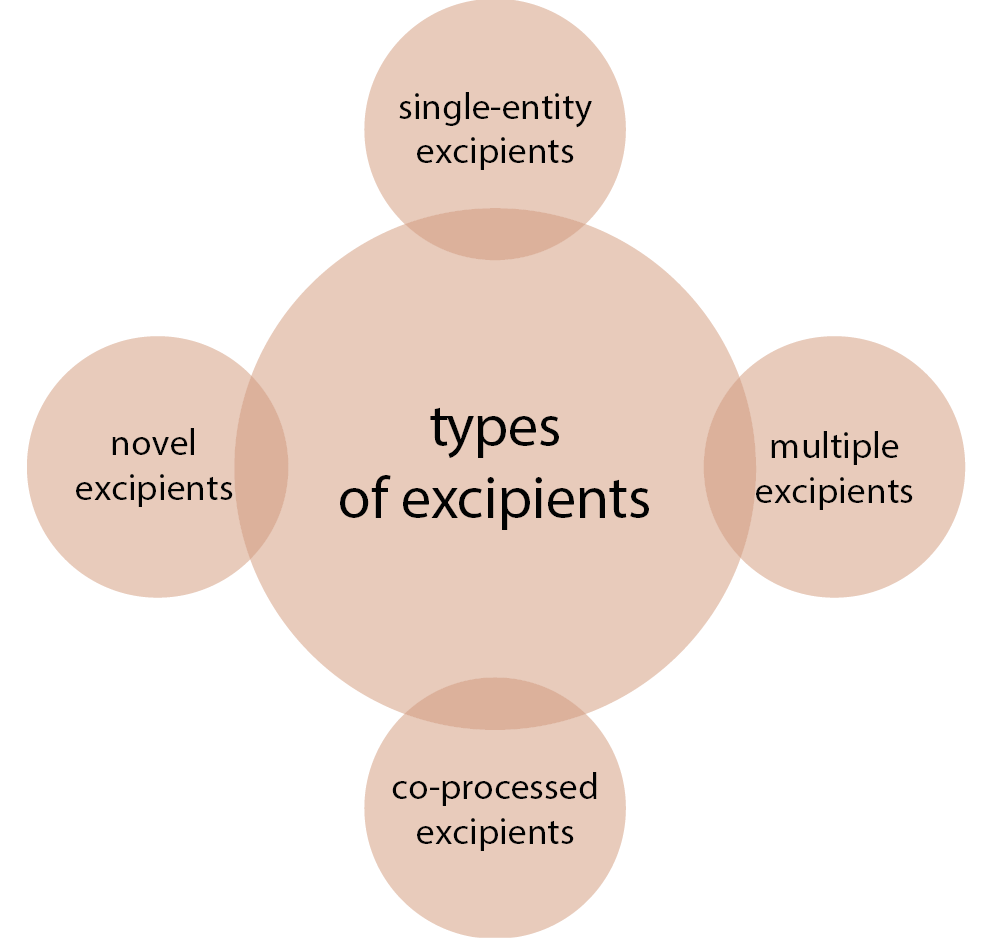
Figure 3 illustrates different types of pharmaceutical excipients used in the preparation of solid dosage forms. Each of these excipients is defined as a single-entity excipient consisting of 1 characteristic component that acts as a primary principal component for the excipients, for example cellulose. Multiple excipients are a blend of 2 or more excipients developed using low to moderate shear force. Under applied pressure, individual constituents are combined without any considerable changes in chemical characteristics, and the individual excipients remain distinct at a particulate level, for example MCC + lactose.34 Novel excipients are excipients that undergo chemical modifications to establish a new excipient. Modifications lead to improvement in solubility and permeability, and result in overall performance enhancement.35 Co-processed excipients are a blend of 2 or more compendia/non-compendia excipients intended to physically alter their characteristics without any significant alteration in their chemical properties. Co-processing is achieved with standard techniques such as granulation, milling, spray drying, etc.36, 37
Co-processed excipients
Co-processed excipients are a mixture of 2 or more compendia/non-compendial excipients that improves physical characteristics without any significant chemical transformations or displaying multifunctional activity.38 Diverse co-processing methods are used in pharmaceutical industries, such as spray drying, solvent evaporation, crystallization, melt extrusion, and many more.39, 40, 41 Co-processed excipients are developed through inclusion of one excipient into the particle framework of another (second) excipient by employing techniques such as co-drying.42 These excipients are designed to address the divergent pitfalls in flowability, compressibility, disintegration potential, lubricant sensitivity, solubility, and permeability, and boost the desired properties of excipients as well as promote production procedure at low expenditure.40, 43
Steps of co-processing
1. Excipient recognition – on the basis of excipient characteristics, attributes, properties, and functionality parameters.
2. Screening of the appropriate proportions of excipients.40
3. Examination of the particle size requisite for co-processing. This step is of utmost importance as when excipient is processed in the dispersed phase, post-processing the particle size of the excipient depends on its initial size.40
4. Electing of an appropriate drying process – for example, spray drying or flash drying.43
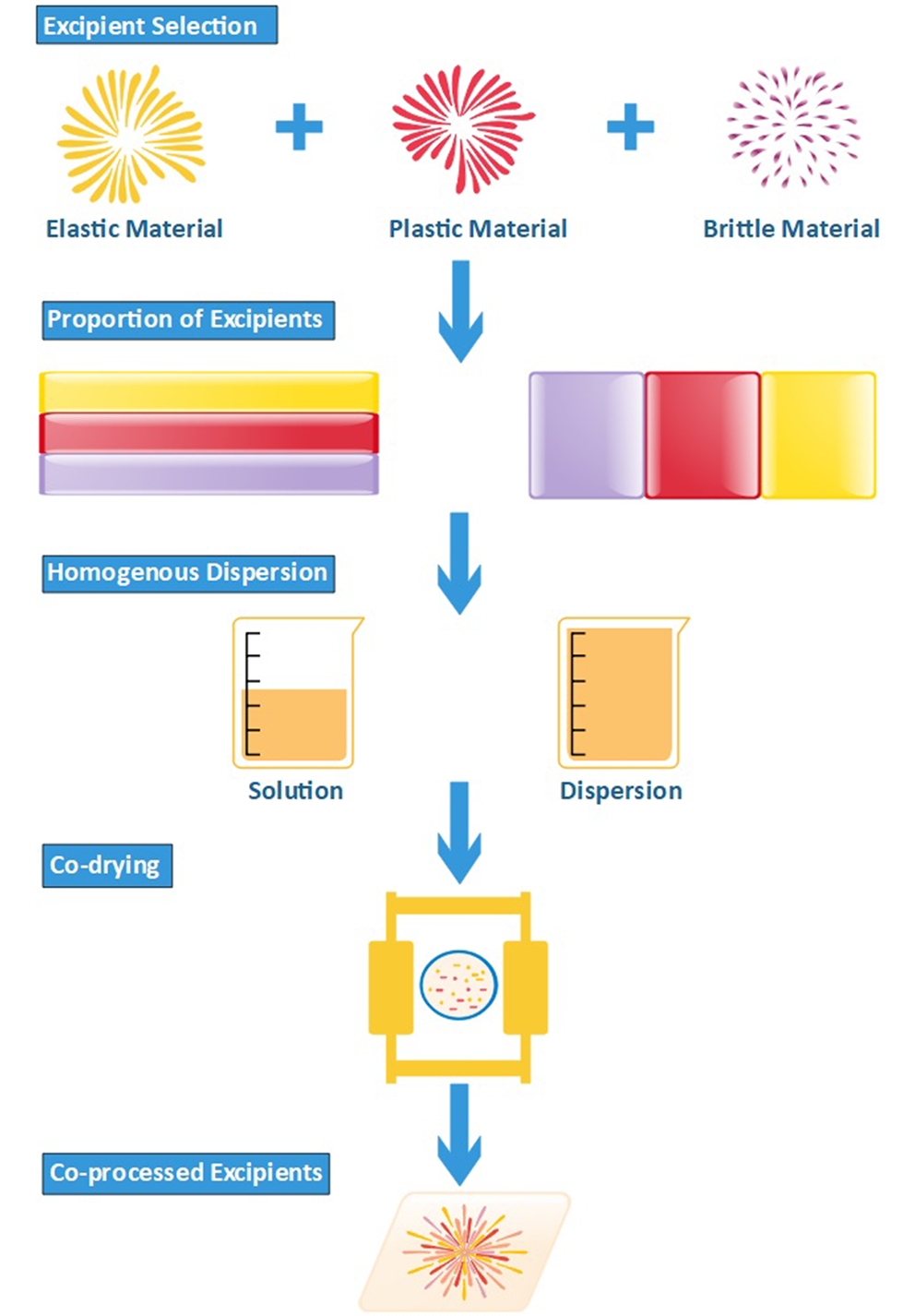
Excipients elected of co-processing should complement each other; for example, mannitol is poorly compressible and a low hygroscopic polyol, and therefore is co-processed with sorbitol that displays good compressibility characteristics as well as high hygroscopicity. Appropriate compression behavior is also required in an ideal tablet excipient, thereby requiring harmony between plasticity and brittleness.44 Figure 4 shows a diagrammatic representation of the co-processing approach.
Co-processing is performed using various other techniques. Table 3 presents the advantages and limitations of each co-processing technique.42, 45, 46, 47, 48
Function of co-processed exciptients in FDT formulations
Co-processed excipients play a vital role in FDT formulations as they improve the flow and wetting properties, modify the compression characteristics, improve the superdisintegration characteristics, and provide superior tabletability. They also enhance thixotropic characteristics by adjusting viscosity, promoting rapid tablet breakdown and contributing to quick therapeutic action (therefore making the tablets appropriate for emergency circumstances).27, 49, 50, 51 Employing co-processed excipients in FDT formulations eliminates the need for any additional excipients or lubricant, and reduces the time and cost associated with FDT production.52, 53, 54
Sunil et al. employed a spray drying technique for the co-processing of MCC, mannitol and aerosil in varying ratios. The prepared excipients were evaluated for different parameters, such as Carr index, Hausner ratio and angle of repose to determine the flow characteristics of the material. The developed co-processed excipients displayed better flow properties compared to a physical admixture of such excipients.55 Pituanan et al. prepared the co-processed pre-gelatinized cassava starch (PCS) with acacia gum (AG) in different proportions using a direct compression method. The developed co-processed excipients were examined regarding their morphology, flow characteristics and moisture content. The result revealed that FDTs prepared using these co-processed excipients exhibit reduced wetting and disintegration time, and altered friability and hardness.56 The FDTs of montelukast were formulated by co-processing mannitol and sodium starch glycolate with the incorporation of a solvent evaporation process by Kumar et al. The prepared formulation was evaluated for hardness, thickness, drug content uniformity, and drug release properties. The results reported that the developed formulation displayed reduced wetting and disintegration time.57 Rao et al. prepared atorvastatin FDTs using novel co-processed excipients crosscarmellose sodium (CCS) and sodium starch glycolate (SSG) in different proportions by employing a direct compression technique. The developed formulation displayed shorter disintegration time in comparison to individual excipients.58 Chlorpromazine HCl orodispersible tablets were designed by Deshmukh et al. using an admixture of excipients, SSG and crospovidone (CP) in varying ratios with a direct compression technique. The developed co-processed excipients were examined regarding their flow characteristics, Carr index and Hausner ratio. The developed FDTs of chlorpromazine exhibit reduced wetting and disintegration time, and enhanced patient acceptance.59
Omeprazole FDTs were formulated by More et al. using co-processed CCS and CP. Carr index, angle of repose and Hausner ratio of developed co-processed excipients were evaluated. The formulated orodispersible tablets exhibited improved drug release characteristics.60 The spray drying method was used by Shirsand et al. for co-processing mannitol and microcrystalline cellulose in various proportions for developing glibenclamide FDTs. The co-processed excipients were then evaluated; the FDTs formulated using an admixture of excipients showed better stability and improved drug release properties.61 Pusapati et al. formulated atorvastatin calcium tablets by employing a direct compression technique using co-processed acacia-calcium carbonate (CaCO3). The hardness, dissolution profile and friability of those tablets were examined. Results revealed that the formulation containing 3% acacia showed less dissolution time and proved to be the overall best formulation.62 Irbesartan FDTs were formulated using co-processed excipients by Madhvi et al. employing a melt agglomeration technique; the bitter taste of a drug was masked using aspartame or by complexing with β-cyclodextrin. The developed tablets were examined for friability, hardness, strength, and dissolution profile. The results reported that melt agglomeration was a better choice for designing FDTs using novel co-processed excipients.63
Table 4 presents a brief outline of the studies conducted on FDTs manufactured using co-processed excipients.50, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76 In continuation of the studies concerning co-processed FDT, several patents are discussed below.
A co-processed mixture of sugar alcohol (such as mannitol) and MCC was patented in 2015. This composition displayed reduced lubricant sensitivity and a better compaction profile, and is widely employed in the preparation of pharmaceutical dosage forms.77 Admixture of pharmaceutical co-processed excipients MCC, polacrilin sodium and partially pregelatinized starch were patented and utilized in the formulation of ibuprofen tablets using a direct compression technique, as the admixture of excipients displayed better flowability and improved compressibility.78 A co-processed admixture of xanthan gum and guar gum excipients was used in the preparation of venlafaxine tablets. Such a combination of pharmaceutical excipients was patented and serves as a bulking, disintegrating, swelling, and gelling agent.79
There are various patents filed for FDTs which utilize co-processed excipients – Table 5 presents a selection of those patents and patent applications. Many co-processed excipients have been introduced into the market and are commercially available.77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89 Table 6 lists commercially available FDTs prepared using co-processed excipients.40, 90, 91, 92, 93, 94
Conclusions
The FDTs are extensively used because of their quick disintegration time and rapid onset of action. Excipients play a vital role in the formulation of tablets and their therapeutic action. A variety of excipients are used to produce FDTs, so there is always a need to search for an excipient with improved functionality that can cater to the high demand for excipients in the pharmaceutical industry. A co-processed excipient is a solution that reduces the use of excessive excipients in the formulation and results in a cost-effective manufacturing process. Co-processed excipients display improved properties in comparison to individual excipients. They reduce the use of excipients in the formulation, thus reducing the overall concentration of the dosage form. Despite having these benefits, co-processed excipients entail certain challenges in terms of their recognition in pharmacopoeia. Hence, extensive research is ongoing in this field and several patents have been filed for co-processed excipients, but there is still no standard characterization for these in the pharmacopeia. Considering the benefits that co-processed excipients provide, the future for such excipients is very promising. A new combination of excipients and improved techniques of co-processing would gain more attention from researchers and the pharmaceutical industry.