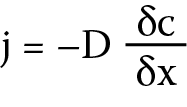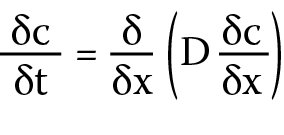Abstract
In recent years, there has been a great interest in the potential use of contact lenses as eye drug delivery systems. Static (individual layers of the cornea, sclera and retina) as well as dynamic barriers (blood flow) pose a serious challenge to the effective delivery of the drug substance to the eyeball. The current ophthalmic systems are not optimal for patients, especially in the form of eye drops, where almost 95% of the drug contained in them is lost through the process of absorption through the conjunctiva or tear drainage. This article describes in vitro experiments that examined the use of contact lenses in the context of drug treatment in infectious, inflammatory, allergic, and glaucomatous diseases. Various techniques used to modify the materials as well as their impact on drug release kinetics were discussed. It has also been demonstrated that these methods can be used in practice during in vivo research, both in animal models as well as in sick and healthy people. The advantages of using controlled-release drug systems in the form of contact lenses are the drug dosing regimen, bioavailability and the prolonged residence time of drugs in the eyeball.
Key words: biopolymers, contact lenses, α-tocopherol, polymeric materials, ophthalmic drug release
Streszczenie
W ostatnich latach widoczne jest duże zainteresowanie potencjalnym zastosowaniem soczewek kontaktowych jako systemów dostarczania leków do gałki ocznej. Bariery statyczne (poszczególne warstwy rogówki, twardówki i siatkówki) oraz dynamiczne (przepływ krwi) stanowią poważne wyzwanie dla skutecznego dostarczania substancji leczniczej do gałki ocznej. Stosowane obecnie systemy okulistyczne nie są optymalne dla pacjentów, zwłaszcza w postaci kropli do oczu, gdzie prawie 95% zawartego w nich leku jest tracone poprzez proces wchłaniania przez spojówkę lub drenaż łzowy. W artykule opisano eksperymenty in vitro, w których badano zastosowanie soczewek kontaktowych w kontekście farmakoterapii w chorobach zakaźnych, zapalnych, alergicznych i jaskrowych. Omówiono techniki zastosowane do modyfikacji materiału oraz ich wpływ na kinetykę uwalniania leków. Wykazano również, że metody te mogą być stosowane w praktyce podczas badań in vivo, zarówno na modelach zwierzęcych, jak i u osób chorych i zdrowych. Zalety stosowania systemów leków o kontrolowanym uwalnianiu w postaci soczewek kontaktowych to reżim dawkowania leku, biodostępność, a tym samym wydłużony czas przebywania leków w gałce ocznej.
Słowa kluczowe: soczewki kontaktowe, biopolimery, α-tokoferol, materiały polimerowe, uwalnianie leków okulistycznych
Introduction
In recent years, there has been a significant development and interest in contact lenses as an alternative way of delivering pharmaceuticals to the eyeball. Commercially available contact lenses are unmodified and release medicinal substances in an uncontrolled manner. Incorporating surface-bound particles, such as liposomes, nanoparticles or deposited diffusion barriers from vitamin E, influences drug release kinetics. Obtaining customized materials for patients may be possible by incorporating functional monomers capable of specific interaction with drug molecules, which result in decreased diffusion rate and molecular printing, modification of the ionic charge of the material, or creation of poly(lactic-co-glycolic acid) (PLGA) drug reservoirs. It is an ever-evolving field, and scientists are developing new methods for assessing drug release and improving existing ones, which gives great hope for future applications.
Publications cover various aspects of the use of contact lenses as drug carriers, such as controlled drug release systems and their clinical use in eye diseases. Holgado et al. discussed the subject of design requirements of lenses, various strategies for their production, and concentrations of drug required to obtain the appropriate release of the drug substance.1 Peral et al. reviewed different techniques of treating glaucoma with the use of lenses with applied drugs.2 Franco and De Marco performed a comprehensive review of several methods employing contact lenses as controlled drug release systems.3 Thanks to this approach, it was possible to obtain information on most of the techniques in the context of many diseases.
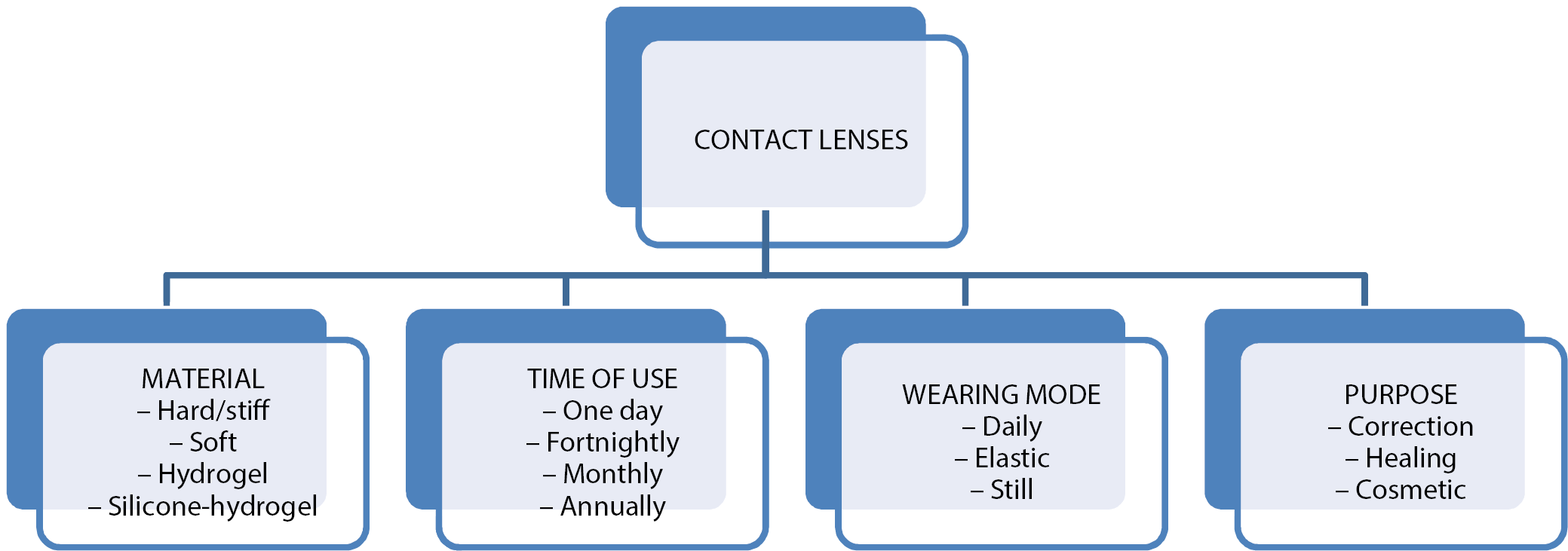
Contact lenses are an example of a commonly used polymer biomaterial. They are divided into different groups based on several main criteria: material, size, geometrical features, indications, and methods of use (Figure 1). From the material point of view, the most commonly used soft contact lenses are divided into 2 basic groups: hydrogel (Hy) and silicone hydrogel (Sil-Hy). They differ regarding the chemical structure of the used materials and their properties. Hydrogels, due to the prefix “hydro”, are identified as water gels, but they should be called synthetic material that swells in water or biological fluids. The Hy is spatially cross-linked and consists of hydrophilic natural polymers such as chitosan and sodium alginate, which can absorb large amounts of water while maintaining their three-dimensional structure. In the Hy, the transport medium for the diffusion of substances is water, while the degree of cross-linking of the matrix affects the possibility of their transport through the material.4, 5 Silicone hydrogel lenses are different from the other groups of materials used to make contact lenses. Combining hydrophobic monomers based on silane units with hydrophilic monomers, such as 2-hydroxyethyl methacrylate (HEMA), was a challenge for researchers due to the phase separation of the individual components and the disturbance of optical focusing, which resulted in blurry vision. Various synthetic methods have been developed, leading to 3 generations of Sil-Hy contact lenses. In generation I, the first approved Sil-Hy materials for contact lenses were lotrafilcon A and balafilcon A, which used phase separation to completely separate the hydrophilic and silicone phases. Lotrafilcon A, belonging to the US Food and Drug Administration (FDA) group, is a mixture of fluoroether macromer and tris hydroxymethyl aminomethane (TRIS), which forms a low-hydrated and non-ionic material with a Dk (oxygen permeability through the lens) coefficient 140. Balafilcon A is the only FDA group III Sil-Hy lens. The material of this lens is manufactured primarily with the use of technologies based on modified TRIS structures. It is a hydrated ionic lens with a Dk of 99. Lenses from generation II consisted of a combination of hydrophilic monomers and made use of macromeric technologies. These materials, galyfilcon A and senofilcon A, combine various monomers and macromers with an internal wetting agent (polyvinylpyrrolidone (PVP)). Galyfilcon A has a Dk of 60, while senofilcon A has a Dk of 103. In generation III, the newest filicon 3 material was created, which was not based on TRIS or PVP, and the only source of silicon were the macromers. This lens has a Dk of 128, which is higher than that of similar lenses (Table 1).6
Witcherle and Lim (German patent DE1084920B) first proposed the use of soft contact lenses as drug delivery systems to the eyes, and taking into account the 1960s patent for Hys applied to the eyeball as contact lenses.7 Currently, the European patent databases contain 1052 patents for contact lenses and 1073 patents for drug release systems. The interest in using drug release systems with contact lenses grew along with the development of the field of biomaterials and bioengineering.7 Such interest is fully justified, as contact lenses are probably the most popular commercially used biomaterial. This is evidenced by high biocompatibilty of contact lenses, as well as willingness to use them among patients.8, 9 The goals of drug therapy to achieve therapeutic drug levels are also clear.10 Eye drops and ointments, which are the most commonly used forms of eye treatment today, do not provide patients with the necessary pharmacological limit. They are limited by their bioavailability and the number of active ingredients reaching the site of action, which results from the corneal absorption barrier, as well as unproductive absorption by the conjunctiva and dilution of the preparation with tears.11 The challenge for researchers is the compatibility of the lens material with the drug, especially in the treatment of chronic diseases such as glaucoma. It is estimated that the treatment of glaucoma with eye drops is effective in only 50% of patients. This effectiveness decreases with the frequency of using the drops and the duration of their application.12 Proper lens design can provide effective dosing over the expected lifetime, which will eliminate frequent drug dosing and carrier replacement. Many methods of determining and modifying characteristics of drug release from contact lenses have been investigated.13 This paper will review the methods used to properly modify the lens for the controlled release of drugs, as well as the possibility of their use in the delivery of drugs to the eye in diseases such as eye infection and inflammation, allergy, and glaucoma.
Commercial contact lenses
as drug delivery matrices
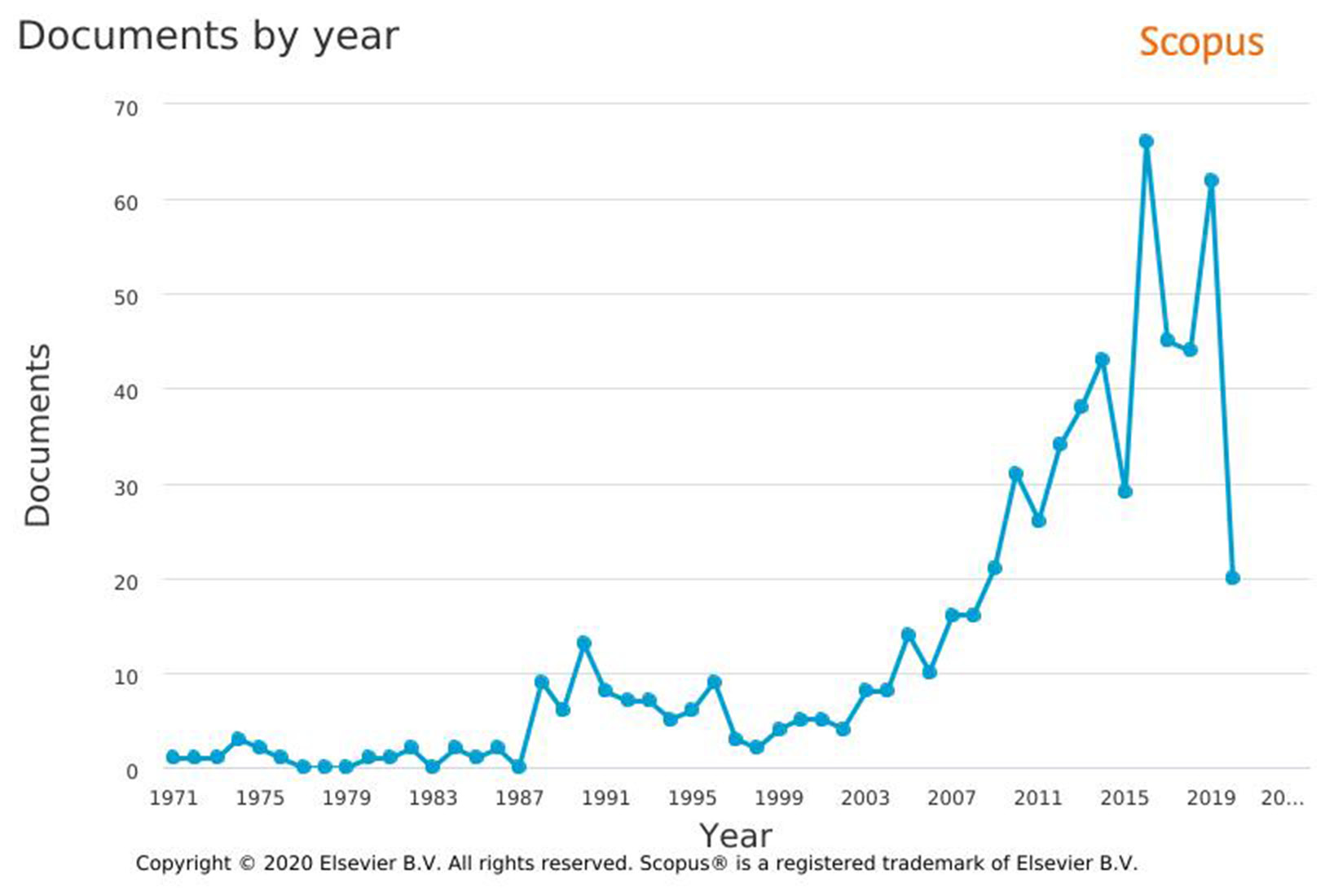
The use of commercially available, unmodified contact lenses for testing gives the opportunity to obtain results for a material with a specific chemical composition. The test follows immediately after their application to the simulated eyeball and allows one to assess their surface during the simulation of the local treatment in a patient. An additional advantage of these lenses is that their use to model the possibilities and kinetics of drug release allows for consistent results due to their large-scale availability and repeatability of the tested product. The factors affecting the release of drugs in many studies conducted on commercially available contact lenses, which use various medicinal products such as dexamethasone3 or ciprofloxacin,14 are the material of the skeleton of the lens (the relationship between Hy and silicone), the equilibrium water content in the lens, and the contact surface with the eyeball. The topic of commercially available contact lenses as systems for the controlled release of ophthalmic drugs has been discussed in recent years. Search in the Scopus database for the phrase “contact lenses” AND “drug delivery” resulted in 642 documents in the period from 1971 to 2020, with intense growth in the number of works since 2003. At the same time, search in the Science Direct database for the same joined phrase resulted in 11,608 documents released until 2020 (Figure 2). Contact materials can absorb and release a significant amount of drug substance in a direct soaking process, but the release kinetics are fast and uncontrolled, and therefore, unsuitable for a long-term drug delivery.3, 12, 13, 15
Modifications of commercially available contact lenses: diffusion barriers using vitamin E
Taking into account the aforementioned pharmacokinetic limitations of commercial contact lenses, attempts have been made to modify these materials in order to obtain the desired properties – in particular to decrease the drug release rate.16 It is assumed that the drug release from the lens is a diffusion-controlled process. According to Fick’s laws (Equation 1), the speed is influenced by both the ability of a given drug to diffuse through the material and the length of the path that must be followed. Diffusivity is a constant value, especially when bonding a lens to a drug, therefore the change in drug release time can be modified by increasing the thickness of the material.17
 (1)
(1)
 (2)
(2)
where:
j – flux of the diffusing component in the x direction
[g/cm2∙s], c – concentration of the component in the flow plane [g/cm3], δc/δx – concentration gradient of the diffusing component perpendicular to the flow plane, and D – diffusion coefficient [cm2/s].
Fick’s law (Equation 1) describes the relationship between the flow of the diffusing substance (i.e., the amount of flowing substance in a unit of time through a unitary surface perpendicular to this stream) and the concentration gradient. Fick’s second law (Equation 2) describes the relationship between the local rate of change in the concentration of the diffusing substance and its concentration gradient. The experimentally determined coefficients of drug diffusion through the lens layer are presented in Table 1.
One way to reduce the rate of drug release is to use a hydrophobic liquid that was incorporated as a component of the contact lenses. One of them is α-tocopherol (α-TOC – vitamin E), which has a low water solubility and is biocompatible with the eye surface.18 The α-TOC (Figure 3) has antioxidant properties, and exerts a positive effect on the eyeball by ensuring the tightness of its cell membranes and the inhibition of cataract development.19 Placing contact lenses in the α-TOC solution after the sorption of the drug leads to the formation of a hydrophobic diffusion barrier on them. This allows for a stable release of the therapeutic dose for a longer time than in the case of traditional dipping.20 The usage of α-TOC causes a slight decrease in oxygen absorption but to an acceptable level that does not affect the wettability of the lens surface. Moreover, the use of lenses with a hydrophobic diffusion barrier facilitates the treatment of bacterial keratitis.21
Both Sil-Hys and pure Hys are commercially available in the form of contact lenses. Attempts have been made to modify them by creating a vitamin diffusion barrier on their surface.22 This led to a significant extension of the drug release time, from several hours for unmodified lenses to several weeks after modification. With the appropriate drug–lens combination, a Sil-Hy lens release time of 3 h was achieved due to drug release time of 10 min for unmodified lenses.23 Barrier formation was achieved by soaking the lenses in a vitamin E solution with ethanol; the composition of the barrier may vary depending on the desired amount of vitamin E in the final product. The advantage of this method is the biocompatibility of both components, acceptable optical properties after modification, and a certain degree of protection against ultraviolet radiation provided by the coating obtained. However, the durability of vitamin barriers and the long-term use of such lenses is unknown. Thus, there is no information as to whether the function of such a barrier is preserved or destroyed during the cleaning of long-term lenses.24, 25
In patients with bacterial and fungal keratitis, the standard treatment consists of administering antibiotics to the conjunctival sac. The frequency of administration (every 30–60 min) is very bothersome. Paradiso et al. combined an in vitro experiment with a mathematical model to develop soft contact lenses for treating keratitis through a prolonged release of appropriate drugs. The focus was on extending the release time of levofloxacin (LVF) and chlorhexidine from 1-Day Acuvue® TrueEye™ and Acuvue® Oasys™ lenses by introducing a vitamin E diffusion barrier. The weight of drugs loaded into the lenses can be controlled to achieve comparable daily release with commonly recommended eye drop therapy. Vitamin E lenses have retained all key properties for in vivo use. Paradiso et al. soaked Sil-Hy lenses in a solution of vitamin E dissolved in ethanol (42 mg/mL) for 3 h. They obtained 32 h of the release of the antibiotic LVF in lenses without vitamin E content, which was extended to 100 h after storage of the lenses in α-TOC solution. This effect has been attributed to the presence of vitamin E nanoaggregates formed in the lenses that have a barrier effect on the release of the drug. The injection of vitamin E into commercial silicone contact lenses did not cause changes in transparency, wettability and ion permeability.26
Hsu et al. developed contact lenses for sustained simultaneous release of timolol and dorzolamide. Co-administration of timolol and dorzolamide increased the release time of both drugs. In addition, the process of releasing both drugs was extended to about 2 days due to the formation of a diffusion barrier from vitamin E. This procedure is very effective in providing a combination therapy involving the combination of drugs with vitamin E.27
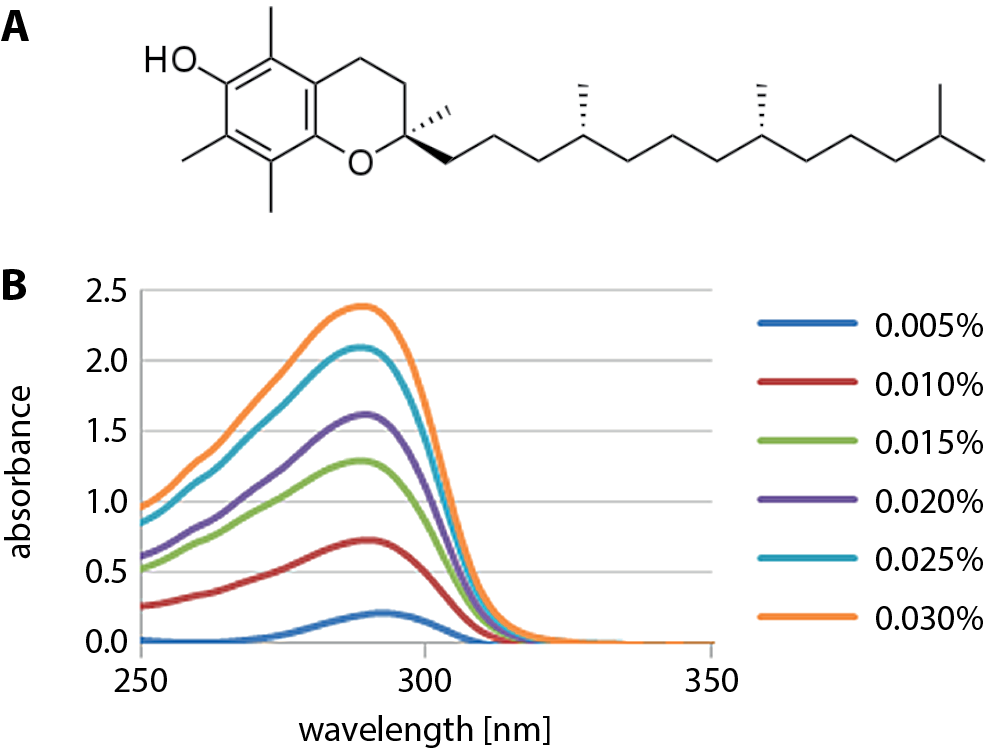
Another group synthesized contact materials by following the production procedures of one of the companies. The resulting Hys were dried at room temperature. The finished material was immersed in 1.5 mL of the vitamin and ethanol solution for 24 h, while the vitamin concentration was maintained in a range of 0.00–0.28 M. After conditioning, the lenses were washed with ethanol and deionized water to remove excess vitamin from their surface. For sorption of the drug with the vitamin inside the lenses, they were immersed for 24 h in 1.5 mL of a solution that contained 0.5 mg of the drug, ethanol and vitamin. Timolol and brimonidine release tests from contact lenses without a diffusion barrier and with a barrier were performed. The concentration was monitored by measuring absorbance at the wavelength of absorption characteristic for the respective drug (λmax = 294 nm for timolol, λmax = 249 nm for brimonidine). Contact lenses loaded with vitamin E significantly increased the total amount of released timolol and brimonidine by 19.1% and 18.7%, respectively, compared to the lens without a barrier layer. These results also suggest that the simultaneous use of vitamin E increases the number of absorbed drugs.28
The results presented by Cheng Chun et al. clearly show that the use of vitamin E in commercial silicone lenses can significantly increase the release time of hydrophilic drugs without affecting the transparency of the matrix. At the same time, a significant reduction in ionic permeability (50%) and a slight reduction in oxygen permeability (45%) were noted, but these reductions were not sufficient to exclude the use of vitamin E in commercial lenses. The mechanism of prolonging the release time of the drug is due to the barrier effect of vitamin E. It is reasonably assumed that the effect is caused by the presence of vitamin E molecules; however, it is also possible that vitamin E does not form macroscopic aggregates and is simply adsorbed on a polymer gel. Surface adsorption may impede the surface diffusion of the drug over the lens surface, leading to a reduction in diffusion rate. Differences between the effects of vitamin E on ion transport and oxygen suggest that vitamin E aggregates can only form in the hydrophilic Sil-Hy lens channels.29
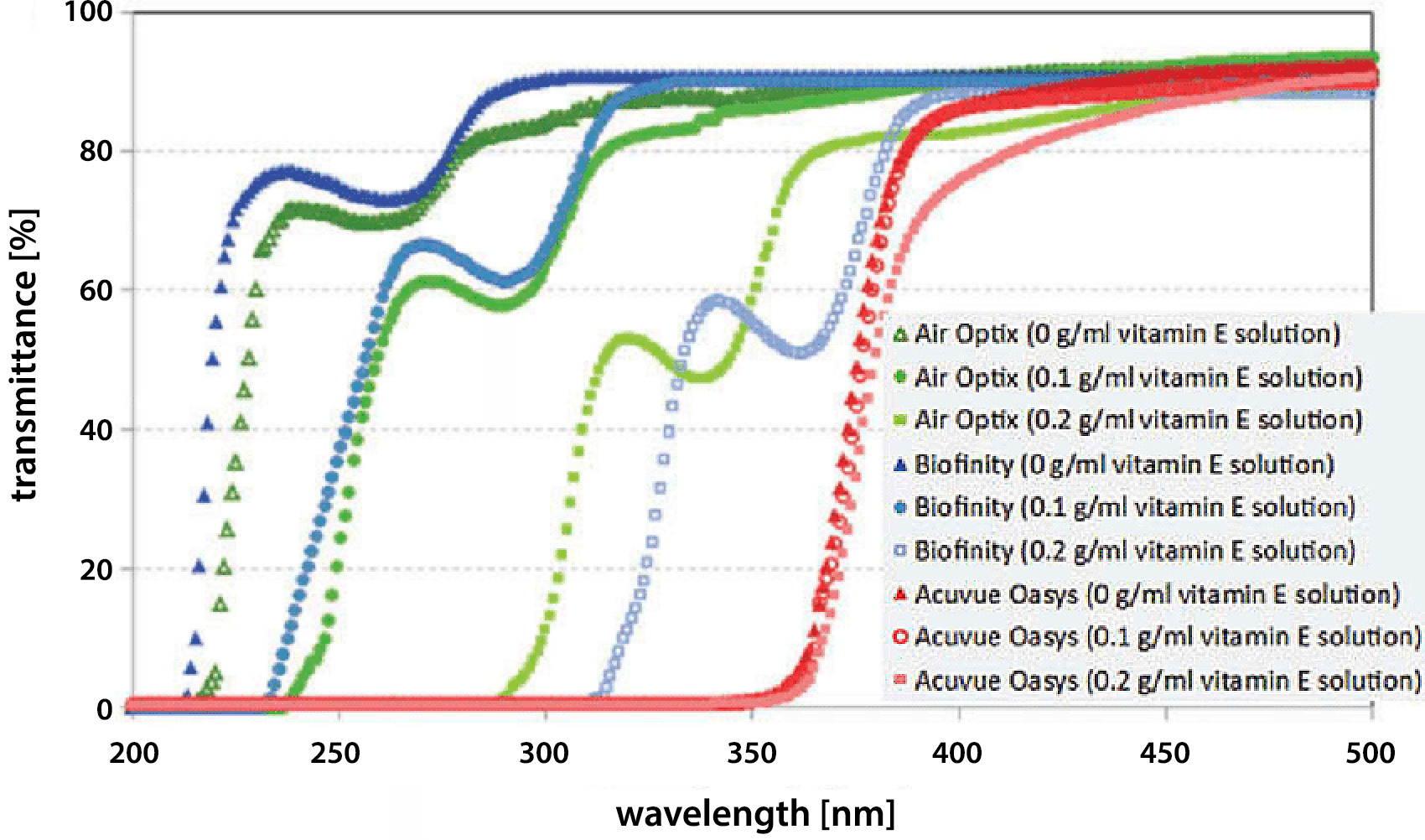
The work of Rad and Mohajeri showed the capability of modified contact lenses to prevent ultraviolet (UV) radiation exposure. Soaking the lenses in vitamin E solution (0.1 g/mL or 0.2 g/mL) shifted UV transmission edge of the spectra to the longer wavelengths in corresponding contact lenses Air Optix and Biofinity (Figure 4). It was shown that UV radiation could be blocked by using such a barrier on the surface of the lenses. Moreover, the data proved that vitamin E loading did not influence the visible light transmittance (400–700 nm) in the studied series, hence the transparency of lenses was still acceptable.30
Drug delivery using novel techniques: molecular printing
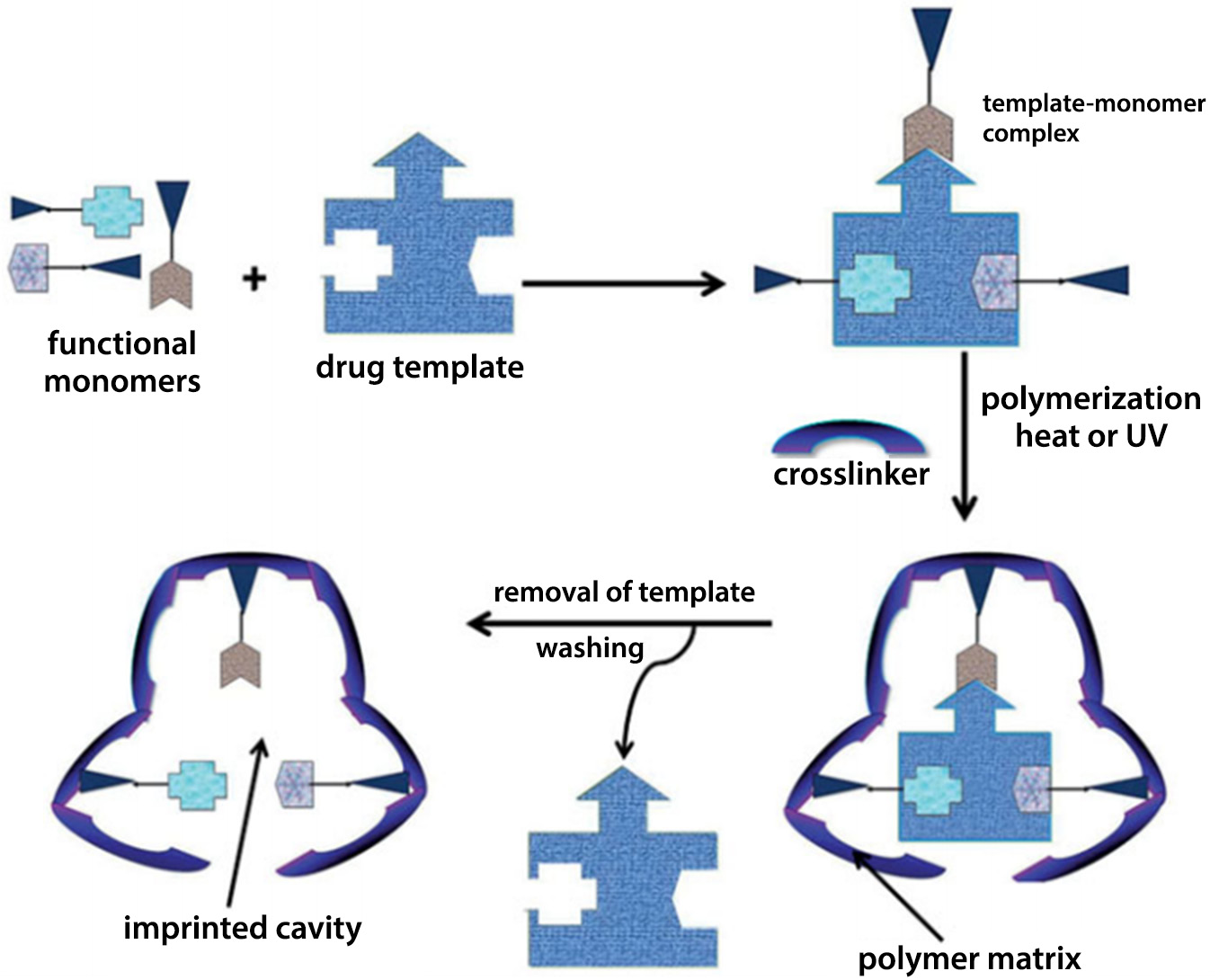
Molecular printing is one of the most widely studied methods in which lens materials are designed specifically for the sustained and controlled release of drugs into the eyeball.31 This method is a type of forced ordering on the surface of a polymer that generates areas complementary to the shape and functional groups of the molecule that is to be originally absorbed (acting as a template) and then released from the matrix. This is achieved by incorporating functional monomers into the matrix that are designed to interact with standard molecules, usually drugs.32 After the polymerization process, the areas designed for interaction form a kind of molecular scale template that allows the diffusion to be significantly slowed down.33 By conducting numerous experiments on the release of the drug substance, it was possible to determine the duration of release, as well as synthetic parameters such as drug concentration or type of polymeric crosslinker.34 This technique has evolved thanks to the knowledge about interactions occurring between human receptors and individual drugs. Such knowledge became the basis for designing the structure of monomers, which allowed the creation of polymer matrices imitating natural receptors with whom the drug interacts in the patient’s body. There is a limit on the amount of substance that can be printed if the polymer to still to function as a contact lens.35
In their review article, Tashakori-Sabzevar and Mohajeri described soft contact lenses with molecular printing (Figure 5), which is shown to be a comprehensive and effective method of optimizing drug release. Such systems show unprecedented control over prolonged loading and release of the drug. In recent years, only a few in vivo studies have been published on the release of the drug into the eye from printed lenses. Soft contact lenses are comfortable and biocompatible. However, the ability to print the drug in a conventional lens is quite low, and therefore, the therapeutic concentration is difficult to achieve.35
In studies conducted by Tieppo et al., the Hy with molecular printing was prepared as an innovative method of drug administration. The concentration profile of the drug-printed lens was compared with unprinted matrices and conventional eye drops in the eyes of New Zealand white rabbits. The results indicated a sustained release of ketotifen fumarate from printed lenses, a constant concentration of 170 lg/mL in tear fluid, and significantly higher bioavailability for 26 h. Maximum drug concentrations with molecularly imprinted polymer (MIP), non-imprinted polymer (NIP) and eye drops for tear fluid were 214 µg/mL, 140 µg/mL and 143 µg/mL, respectively. The maximum concentration times were 240 min, 60 min and 0 min for MIP, NIP and eye drops. In addition, the ability of MIP lenses to accumulate drugs was almost 3 times higher than in NIP. The future of contact lens printing is very promising and requires further clinical and preclinical research. Further advances in polymer engineering and the design of intelligent binding sites in Hys can lead to remarkable advances in the preparation of new drug delivery systems for the eye.36
Dry eye syndrome is caused by a disruption in the tear film, which leads to abnormal hydration of the eye. This may be due to its incorrect composition or excessive evaporation, which results in the drying out of cornea and conjunctiva.37 Soft contact lenses can relieve the symptoms of dry eye by slowly releasing the agent that keeps the eyeball moist. Sil-Hy lenses with sustained, controlled release of hydroxypropyl methylcellulose using a molecular printing strategy were manufactured. The commercial Sil-Hy lens was adapted to release approx. 1000 μg of the drug for up to 60 days continuously at a rate of 16 μg/day at physiological flows, releasing an equal portion of the drug throughout the use of the matrix. Release rates can be significantly affected by the print effect and the functional weight ratio of the monomer to the template (M/T). The M/T values of 0, 0.2, 2.8, and 3.4 correspond to the release times of 10, 13, 23, and 53 days, respectively. The Sil-Hy lenses had high optical quality and adequate mechanical properties for patient use. This work highlights the potential of printing in the design and engineering of Sil-Hy lenses to release macromolecules during wear, which can reduce the symptoms of dry eye.38
The goal of the next work was to develop an innovative, supercritical fluid-assisted molecular printing method to give commercial soft contact lenses the ability to absorb specific drugs and control their release. This approach aims to overcome the limitation of the widespread use of pre-formed soft contact lenses by the following: immersion in concentrated drug solutions (only possible for drugs with high water solubility), the use of molecular printing methods that require drug selection before polymerization, and the introduction of a drug adapted to the network. Supercritical carbon dioxide (scCO2) impregnation tests were performed at 12.0 MPa and 40°C, while scCO2 extractions were carried out at 20.0 MPa and 40°C. For comparative purposes, conventional experiments of flurbiprofen sorption and drug removal in aqueous solutions were carried out. Soft contact lenses processed with supercritical fluid showed recognition ability and greater affinity for flurbiprofen in an aqueous solution than for structurally related ibuprofen and dexamethasone, suggesting that the formation of a molecular impressed cavities arising through both physical (swelling/plasticizing) and chemical (carbonyl groups in the network with the substituent C-F in medicine) interactions. Processing with scCO2 resulted in no changes in some of the critical functional properties of soft contact lenses (glass transition temperature, transmittance, oxygen permeability, contact angle), enabled the control of the amount of loaded/released drug molecules (by using several successive processing cycles), and allowed the preparation of hydrophobic therapeutic drug-based soft contact lenses in a much shorter process time than using conventional aqueous molecular printing methods.39
In the another study, a series of Sil-Hy with MIP and NIP was prepared using HEMA as a skeletal monomer, ethylene glycol dimethacrylate (EGDMA) as a crosslinking monomer, methacrylic acid (MAA) as a functional monomer, and dorzolamide (DZD) as a standard molecule. Two molar ratios of DZD:MAA (1:8 and 1:4) and 400 mM MAA were also used in the printing process. The Hys (0.4 mm thick) were synthesized by thermal polymerization at 50°C for 24 h in a polypropylene form. Then, the swelling and binding properties of Hys in water were evaluated. Their sorption and release properties were also tested in 0.9% NaCl and artificial tear fluid. The results showed that the use of MAA as a comonomer and the use of molecular printing techniques increased the capacity of Hys. The optimized printed Hy (MIP1:4), prepared with 400 mM MAA and having a molar ratio of DZD:MAA 1:4, had the highest affinity for DZD and the greatest ability to control the release process in an aqueous environment.40
Delivery of drugs from materials prepared in PLGA technology
Lactic and glycolic acid copolymers (PLGA) are one of the most widely studied materials used to deliver drugs to the human body. They are characterized by good biocompatibility and biodegradability, and have been approved by the FDA. These polymers are tested not only for applications in the eyeball but also as tissue scaffolds and implants throughout the body.41 They exhibit high mechanical strength and release rate, which can be controlled by the relative ratio of lactic acid to glycolic acid in the final material.42 The incorporation of several ophthalmic medications in a PLGA matrix on the contact lenses allows the simultaneous delivery of several substances to the patient’s eyeball. In vivo research suggests that this solution may maintain controlled drug release effects for up to a month and longer. However, the use of PLGA technology has disadvantages: even the very thin layers of the components interfere with the translucency of the lens and, in consequence, it does not transmit visible light as effectively as a commercial Hy lens. In addition, an adverse effect is an increase in the total thickness of the lens.43, 44, 45, 46
Ciolino et al. coated PLGA films with the poly(2-hydroxyethyl methacrylate) (PHEMA) through UV polymerization. The HEMA and EGDMA crosslinkers were used as monomers. The solution was photopolymerized to form the bottom layer of a composite contact lens constructed using PHEMA. The PLGA film with a model drug (fluorescein) was manually pressed on a dried PHEMA gel. The drug placed between layers of PHEMA without PLGA film caused a slower release than that of the drug on the PLGA film but faster than that of the drug on the PLGA film coated with PHEMA.47
Subsequent studies investigated experimental parameters such as the composition of polymer mixtures, the type of stabilizer and the amount of active pharmaceutical ingredient in the production of polymer drug delivery systems for topical administration of prednisolone to the eye. To achieve this goal, PLGA nanoparticles with prednisolone were prepared by solvent evaporation. Prednisolone was quantified using a validated high-performance liquid chromatography (HPLC) method. The amount of PLGA copolymer had the greatest impact on the size of the nanoparticles, while the amount of used prednisolone had the greatest impact on the drug loading. Longer homogenization time with more prednisolone resulted in the smallest size nanoparticles. The produced nanoparticles had an average particle size of 347.1 ±11.9 nm with a polydispersity index of 0.081. The nanoparticles were then introduced into the solution before polymerization. Bright and transparent matrices with high drug encapsulation efficiency (EE) in a range between 60% and 92% were successfully prepared. When nanoparticles (NP) with contact materials were compared with control contact lenses (NP-unloaded), there was a 2% decrease in hydration and an 8% decrease in light transmission. The contact lens wettability remained within the desired range of values (<90°C) even after the introduction of NP. Both NP and NP-unloaded lenses showed a slow release of the drug in vitro over 24 h.48
Supply of medicines
from charged materials
Many ophthalmic drugs have an ionic charge that can be used to increase absorption and release. The FDA classification of contact lenses is based on the presence or absence of surface charge. This helped predict the deposition of tear components and the antiseptic characteristics of these materials when used with different cleaning solutions.49 The surface charge of these materials also had an impact on the uptake and release of pharmaceutical products.50, 51 The number of anionic molecules such as methyl methacrylate (MMA) was increased to improve the sorption properties of these materials, which allowed a change in charge and increased attraction of charged drug molecules.52 However, there are concerns about the surface charge of these molecules as this may increase the tendency for deposition of tear film components (such as proteins) on the lens surface, especially concerning long-term ones.53
Christopher and Chauhan tested the effect of placing electrodes on contact lenses to obtain an electric field gradient for transporting ionic drugs to the cornea. Commercial lenses, loaded with Nile blue and fluorescein as hydrophobic and hydrophilic drug analogs, respectively, were placed on eyeballs of rabbits. The electric field gradient was generated by placing the cathode and anode on the lens diametrically opposite each other. Electric power showed an increase in uptake of Nile blue, and quantity was a function of duration and current intensity. Similar increases in flow were observed for fluorescein. Images recorded with confocal microscopy also showed an increase in dye penetration in the presence of current. Connecting electrical current to the lens can be a minimally invasive and effective approach to obtaining a therapeutic dose of the drug delivered. The results of ex vivo experiments suggest that a contact lens containing both cathode and anode may be useful for delivering drugs to the eye using iontophoresis techniques. Since the distribution of the electric field can be accurately predicted, it is possible to obtain a smaller, easy-to-use device, which may result in higher bioavailability and allow for predicting the delivered dose of the drug. In addition, a compact device would be much more convenient for patients than other devices due to the smaller and more localized electric field, and could be worn like a normal contact lens without seriously obstructing the field of view.54
Takamatsu et al. proposed a powering system for an electrical contact lens that is capable of iontophoretically delivering drugs to the eye.55 A hybrid power generation device is comprised of a wireless power transfer system and a bioabsorbable metal–air primary battery. No hydrogen evolution or pH change was observed in the tear electrolyte, hence it was proposed that the shown power source could power wearable electronics in body fluids.56
Zhu et al. presented a new lens embedded in the inner layer of the matrix, which was capable of pH-triggered prolonged drug delivery to the eye with good storage stability. Eudragit S100 and ethyl cellulose film were used as the inner layer, while PHEMA Hy was used as the outer layer. Using diclofenac sodium as a model drug, the obtained matrices were examined and optimized in terms of the ratio of polymer in film, viscosity, the ratio of drug to polymer, the thickness of the inner layer, and the thickness of the PHEMA layer. Drug release was triggered by a change in ambient pH which allowed the storage of the contact lens to embed in the inner layer in a phosphate-buffered solution (PBS) at pH 6.8 with an imperceptible loss of drug and slight changes to the drug release pattern. An in vivo pharmacokinetic study in rabbits showed a sustained release of the drug for more than 24 h in the tear fluid, indicating a significant improvement in residence time in the cornea. Correlation between in vivo and in vitro studies (IVIVC) was established between in vitro drug release and in vivo drug concentration in tear fluid. In summary, this design of the embedded contact lens within the inner layer can be used as a platform for sustained delivery of drugs to the eye with translational potential in the treatment of ocular diseases.57
Faccia et al. used pH-sensitive copolymers containing HEMA with different proportions of 2-(diisopropylamino) ethyl methacrylate (DPA) and different amounts of the crosslinker (i.e., EGDMA). These were rated as pH-sensitive drug delivery systems for potential use in ophthalmic therapies. The characterization of the pH response was carried out using swelling studies and scanning electron microscopy (SEM) analysis. Drug loading and release at different pHs were evaluated using Rhodamine 6G (Rh6G) as a model drug. The obtained results showed that the presence of DPA in copolymers confers pH sensitivity properties.58
Contact lenses releasing medicinal substances can be very effective carriers for the delivery of ophthalmic drugs, but usually they are not able to release the drug for more than a few hours. Bengani and Chauhan optimized the interaction of the polymer matrix of the contact lens with hydrophobic fragments of ionic surfactants to adsorb surfactant molecules on a highly packed polymer, and thus created a high surface charge. The ionic drugs then adsorbed onto charged surfaces coated with surfactants with high affinity to reduce the transport speed, which led to a sustained release. In particular, they showed the controlled release of the anionic drug dexamethasone 21-disodium phosphate from PHEMA contact lenses using the cationic surfactant – cetalkonium chloride (CAC). The drug partition coefficient increases exponentially with the gel surfactant charge according to the Debye–Hückel theory.59 In the studied case, the drug was adsorbed on a surfactant-coated polymer and could also diffuse along the surface with a lower diffusivity than that of a free drug. This led to a reduction in effective diffusivity, which was a weighted combination of free and surface diffusion. The addition of the surfactant did not affect the transparency of the lenses, and it had the additional advantages of increasing wettability and significantly reducing protein absorption. A 10% addition of CAC provided a significant reduction in protein uptake for lysozyme serving as the model protein in the in vitro studies of protein interactions with Hys. At approx. 10% surfactant exposure, the release time was increased from approx. 2 h to 50 h in 1-day Acuvue® contact lenses.60
Odrobinska and Neugebauer synthesized amphiphilic copolymers containing HEMA and MMA by controlled radical transfer polymerization (ATRP) using bromoester modified retinol (RETBr) as a new initiator. An analogous series of copolymers with regulated hydrophilic–hydrophobic balance was obtained using a standard initiator, i.e., ethyl α-bromoisobutyrate (EBriB). The hydrophilic/hydrophobic ratio in the copolymer has been indicated as a key factor regulating the efficiency of encapsulation processes calculated at a range of 5–98%. Polymer systems with satisfactory encapsulation properties were obtained. The release release profiles indicated that they are attractive micellar carriers of antioxidants. Due to their activity, they can be used in popular approaches in cosmetology, such as masks, eye patches or compresses.61
Supply of drugs for patients suffering from diabetes
Most treatments for diabetes-related eye diseases are systemic (oral) or intravitreal. The goal of Alvarez-Rivera et al. was to design contact lenses suitable for the local prevention/treatment of diabetes-related eye pathologies. The main idea was to incorporate the functional groups into the polymer matrix that can reversibly interact with epalrestat, an aldose reductase inhibitor. Several sets of Sil-Hy were synthesized and the content of chemicals such as HEMA, monomethacryloxypropyl-sym-polydimethylsiloxane hydroxypropyl terminated (MCS-MC12) and aminopropyl methacrylamide (APMA) has been changed. Epalrestat was included before or after polymerization, and the load and release profiles were compared. All samples were evaluated in terms of optical properties, oxygen permeability, swelling, cytological compatibility, eye irritation, and corneal penetration (using drug solution as reference). At the 1st release stage, efficacy strongly depended on the APMA content, which allowed for the prolonged release of the drug in 0.9% NaCl for 1 week, both after synthesis and after loading. Epalrestate-laden Hys also showed anti-cataract activity in an in vitro diabetic eye model. In general, biologically inspired Sil-Hy contact lenses are the first attempt at designing contact lenses adapted to the needs of diabetic eyes. These act as platforms for the controlled release of epalrestat, promote drug accumulation in the eyeball and stimulate corneal diffusion.62
Methods for contact lenses modification through the use of lipid layers
The use of deposited layers of liposomes or nanoparticles with the absorbed drug affects the delayed release of drugs from contact lenses. The drug is then released from the liposomes or nanoparticles as a result of the interaction with the tear film. The special feature of this method is that the release of drugs from the deeper layers of the lens goes through the previously used layers. In this way, drug release from these materials usually occurs in several phases with different release kinetics: the initial rapid release phase and the stable, slower diffusion of the drug from the inner layers.63, 64
Wang et al. assessed the effect of a 2-week regular application of phospholipid liposomal spray on the thickness of the lipid layer, tear film stability, subjective wearing comfort, visual acuity, and lipid deposition in those wearing Sil-Hy lenses. The phospholipid liposomal spray was applied to the lens of 1 eyeball. On the 14th day of the study, the thickness of the lipid layer and the stability of the tear film increased in the eyes of the treated patients. A larger percentage of participants reported an improvement in sight comfort in the treated eye.65
Danion et al. used in vitro antibacterial evaluation methods for contact lenses containing LVF-laden liposomes, which were designed to prevent and treat bacterial eye infections such as keratitis. The LVF was incorporated into the liposomes before immobilizing intact liposomes on the surface of soft contact lenses using a multilayer immobilization strategy. The release of LVF from contact lenses containing 2, 5 and 10 layers of liposomes into a saline buffer at 37°C was monitored through measuring fluorescence. The release profile of LVF has been described as a mechanism involving 2 independent first-order kinetic models. The total release of LVF from contact lenses was completed within 6 days. The release of LVF from contact lenses containing 10 layers of liposomes and then soaked overnight in LVF solution was also studied and compared with the reference sample. It consisted of pristine contact lenses with no chemical modification that were previously dried and subsequently hydrated in a drug solution. The antimicrobial activity of the liposome-coated contact lenses was assessed by measuring the diameter of the agar zone on the agar plate and optical density (OD). Liposome-coated lenses showed antibacterial activity on both the agar and the OD test after 24 h. When the initial bacterial inoculum was equal to or lower than 106 CFU/mL, all bacteria were inhibited within 2 h. When using an initial bacterial inoculum of 108 CFU/mL, the initial release provided by immersion of liposomal lenses was required for the first hours to inhibit bacterial growth.66
Another research group immobilized multilayer liposomes on soft contact lenses. The liposome skeleton was prepared by exposing NeutrAvidin™-coated contact lenses to liposome aggregates produced by the addition of free biotin to the solution. The X-ray photoelectron spectroscopy (XPS) confirmed immobilization of liposomes, while enzyme-linked immunosorbent assay (ELISA) test showed that neutravidin docking was dependent on biotin-neutravidin affinity binding. The kinetics of fluorescent dye release showed that intact liposomes were immobilized on contact lens surfaces. The stability of the surface-immobilized liposomes on contact lens surfaces was temperature-dependent. Surface-associated liposomes can be stored for up to 1 month at 4°C with little release.67
In another work, liposome-based coatings were analyzed to control the release of the drug from soft contact lens materials. A LVF-loaded PHEMA Hy material was used as a model system for these studies. The coatings consisted of polyelectrolyte layers containing 1,2-dimiristoyl-sn-glycerol-3-phosphocholine (DMPC) and DMPC+cholesterol (DMPC+CHOL) liposomes. The effect of friction and temperature on drug release was studied. The purpose of friction tests was to simulate eyelid blinking to investigate whether soft contact lens materials coated with liposomes can retain their properties, in particular the drug release capacity. It was observed that under the test conditions, the friction did not significantly affect the release of the drug from the liposome-coated PHEMA material. In contrast, an increase in the release temperature led to an increase in the diffusion rate of the drug through the Hy. This phenomenon was recorded in both control and coated samples.68