Abstract
Background. Polymethylmethacrylate (PMMA) is the most used material for the manufacturing of eye prostheses.
Objectives. To investigate the cytotoxicity of different cleaning agents for ocular prostheses on human conjunctival cells.
Materials and methods. Six groups of specimens were created (saline, soap, 4% chlorhexidine, hydrogen peroxide, 1% triclosan, and citronella oil). Three specimens were made for each disinfectant at each disinfection period (1, 7, 15, 30, 60, and 90 days), totaling 108 specimens. Thus, the specimens were disinfected, with different disinfectants, for different periods of time. After each disinfection process, the specimens were washed with sterile distilled water. A human conjunctival cell line was grown on the acrylic resin specimens and then cytotoxicity tests (MTT and Neutral Red (NR)) were performed. A negative control (untreated cell cultures) and positive control (Tween 20) were created. Two-way analysis of variance (ANOVA) and Bonferroni test were performed (p < 0.05).
Results. For the MTT and NR tests, when there was a significant difference between the disinfectant and negative control, the disinfectant generated a significant reduction in cell proliferation most of the time.
Conclusions. All reductions in cell proliferation caused by the disinfectants were clinically acceptable. All disinfectants tested in this study were found to be non-cytotoxic to human conjunctival cells.
Key words: artificial eye, acrylic resins, cytotoxicity, materials testing, conjunctiva, PMMA
Background
Ocular prosthetics are important in the rehabilitation of patients who have suffered ocular loss.1, 2, 3, 4, 5, 6 They can restore facial aesthetics, prevent eyelid deformation, direct and prevent the deposition of tear fluid in the anophthalmic site, protect the mucosa, and help the lacrimal glands partially recover their original position.1, 3, 4, 6 Furthermore, these prostheses can help to increase the self-esteem of anophthalmic individuals.1, 4
Polymethylmethacrylate (PMMA), also known as acrylic resin, is the most used material for the manufacture of eye prostheses, as it makes them more versatile, resistant and comfortable to use.9 Currently, N1 acrylic resin (white color) is among the most used materials for making ocular prostheses due to its similarity to the color of the human ocular sclera, durability, ease of hygiene, and retention in the anophthalmic site.3, 4
Soap, chlorhexidine gluconate, hydrogen peroxide, citronella oil, and triclosan are cleaning agents used to disinfect eye prostheses.5 Although the disinfection of ocular prostheses is essential, the patient, after using the disinfectant, may not adequately rinse their prosthesis before replacing it in the anophthalmic socket. Thus, chemical components can be released into this cavity, potentially causing damage to the mucosa.7
In 2023, a search on PubMed using the terms “conjunctival”, “ocular prosthesis” and “cytotoxicity” showed that there are no articles evaluating the cytotoxicity of disinfectants used to clean ocular prostheses on human conjunctival cells. Thus, this study aimed to evaluate the cytotoxicity of disinfectants (soap, chlorhexidine gluconate, hydrogen peroxide, triclosan, and citronella oil) used for cleaning eye prostheses on human conjunctival cells.
Materials and methods
Manufacturing of acrylic resin specimens
The preparation of the specimens was carried out according to the procedure described by Andreotti et al.5 The thermopolymerizable acrylic resin used was N1 (white color, VIPI STG; VIPI Indústria, Pirassununga, Brazil).4 Polymerization was carried out using microwaves.5 All specimens were circular and had the same dimensions (10 × 3 mm).1, 3, 4, 5
The specimens were polished with abrasive papers of different granulations (280, 320, 600, and 1200; Norton, São Paulo, Brazil; APL-4; Arotec, Cotia, Brazil) under constant irrigation for 3 min (sum of times used for each sandpaper) and, later, with felt and 1-μm diamond solution for 1 min. Next, the specimens were cleaned with distilled water in ultrasound for 20 min. This procedure aimed to remove debris from the surface of the material. Then, the specimens remained on a bench to allow their natural drying.
Groups
The specimens were divided into the following groups:
1) saline group (0.9% NaCl; Apothicario Manipulation Pharmacy, Araçatuba, Brazil) – immersion in 1 mL of saline for 10 min5;
2) neutral soap group (Johnson & Johnson, São José dos Campos, Brazil) – neutral soap has not been diluted with water. Cleaning with this product was associated with rubbing the samples with gauze for 30 s5;
3) 4% chlorhexidine group (Apothicario Manipulation Pharmacy) – immersion in 1 mL of chlorhexidine for 10 min5;
4) Efferdent group (effervescent tablets; Efferdent Original Denture Cleanser; Pfizer Consumer Health, Morris Plains, USA) – immersion in the hydrogen peroxide solution (250 mL of sterile H2O and 1 effervescent tablet = 0.17% H2O2) for 15 min5, 6;
5) 1% triclosan group (Apothicario Manipulation Pharmacy) – immersion in 1 mL of 1% triclosan for 10 min5, 9;
6) citronella oil group (Apothicario Manipulation Pharmacy) – immersion in 1 mL of citronella oil for 10 min5.
Three specimens were made for each disinfectant at each disinfection period (1, 7, 15, 30, 60, and 90 days), totaling 108 specimens. Thus, the specimens were submitted to the processes described above for different time periods (1, 7, 15, 30, 60, and 90 days). Next, the specimens were washed with sterile distilled water for 30 s to simulate a clinical situation.
After disinfecting and rinsing the specimens, the groups were subjected to cytotoxicity testing at each time point.
Cytotoxicity of disinfectants
Specimens from each group were added to a tube with 6 mL of culture medium 199 (Gibco, New York, USA) supplemented with a 5% concentration of fetal bovine serum (FBS), previously determined in a pilot study, and incubated in a bacteriological oven at 37°C for 24 h. Subsequently, Millex filters (0.2 μm; Merck Millipore, Darmstadt, Germany) were used to filter the eluates.1, 3, 4
For the culture and maintenance of cells, the human conjunctival cell line (Wong Kilbourne derivative of Chang conjunctival cell line; clone 1-5c-4), obtained from the American Type Culture Collection (ATCC; CCL-20.2; Manassas, USA), was used and expanded in culture medium supplemented with 10% FBS. Cells were incubated at 37°C in a 5% CO2 environment1, 3, 4 until they reached confluence. Then, 1 mL of cell suspensions of 1×105 cells/mL was pipetted into each well of a plate containing 24 wells.1, 3, 4 After 24 h, the culture medium was discarded, and 500 μL of each previously obtained eluate containing 5% FBS was added to each well (n = 3/group). After incubation, MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide)1, 3, 4 and Neutral Red (NR)10 assays were performed.
The MTT is a colorimetric analysis used to measure mitochondrial metabolic activity.1, 3, 4After 24 h, eluates were replaced with a FBS-free medium containing 0.5 mg/mL of MTT, and incubated at 37°C for 2 h.1, 3, 4 After incubation, the medium was aspirated and 500 μL of isopropyl alcohol was added to each well.1, 3, 4 Absorbances were measured in triplicate at a 570-nm wavelength using a UV-visible spectrophotometer (SpectraMax 190; Molecular Devices, San Jose, USA).1, 3, 4 The NR assay measures lysosomal activity in a culture. After 24 h, eluates were replaced with 0.5 mL of NR dye, and the plates were incubated at 37°C for 2 h. Then, the NR and medium were replaced with 500 μL of 1% formic acid for washing. The formic acid was removed and 1 mL of 1% acetic acid was added to each well. After extracting the dye from the cells, cell viability was assessed in triplicate using the optical density of the resulting solution at 540 nm with a UV-visible spectrophotometer.
For MTT and NR assays, the negative control were cell cultures without any treatment. They consisted of 1 mL of culture medium with 5% FBS. The positive control consisted of medium with Tween 20 (Sigma-Aldrich, Saint Louis, USA), which is a non-ionic detergent responsible for cell lysis in cytotoxicity assays.1, 3, 4 Both were subjected to the same conditions of incubation and temperature used to obtain the eluates (n = 3/group).
Real-time reverse transcription quantitative polymerase chain reaction (RT-qPCR) was performed to quantitatively assess gene expression levels for type IV collagen (COL IV, COL4A3BP: Hs00178621_m1), transforming growth factor β (TGF-β, TGFB1: Hs0099133_m1), matrix metalloproteinase 9 (MMP9, MMMP9: Hs00234579_m1),1, 3, 4 and markers related to apoptosis and cell death that included myeloid cell leukemia sequence 1 (MCL-1, MCL1: Hs01050896_m1) and caspases 3 (CASP-3, CASP3: Hs0023487_m1) and 9 (CASP-9, CASP9: Hs00609647_m1).1, 11, 12
The StepOnePlus Real-Time PCR System (Applied Biosystems, Life Technologies, Foster City, USA) was used.1, 3, 4 The β-actin (ACTB: Hs030223880_g1) was utilized as an endogenous control.1, 3, 4 The results were analyzed with the cycle threshold.1, 3, 4
Cytotoxicity analyses were carried out respecting the norms of the ISO 10993-5 standard for in vitro toxicity analysis.13 Cell proliferation percentages of the controls represented 100% and cell viability (%) was calculated for all groups.1, 3, 4
Statistical analyses
Two-way analysis of variance (ANOVA) and Bonferroni test were used (p < 0.05). The variation factors were disinfectants (saline, soap, chlorhexidine, hydrogen peroxide, triclosan, and citronella oil) and time points (1, 7, 15, 30, 60, and 90 days).
Results
The two-way ANOVA showed a significant relationship between the variation factors (cleaning agents and times) and the results of cytotoxicity assays (MTT, NR and RT-qPCR; p < 0.001 for all factors).
MTT (disinfectant compared to control)
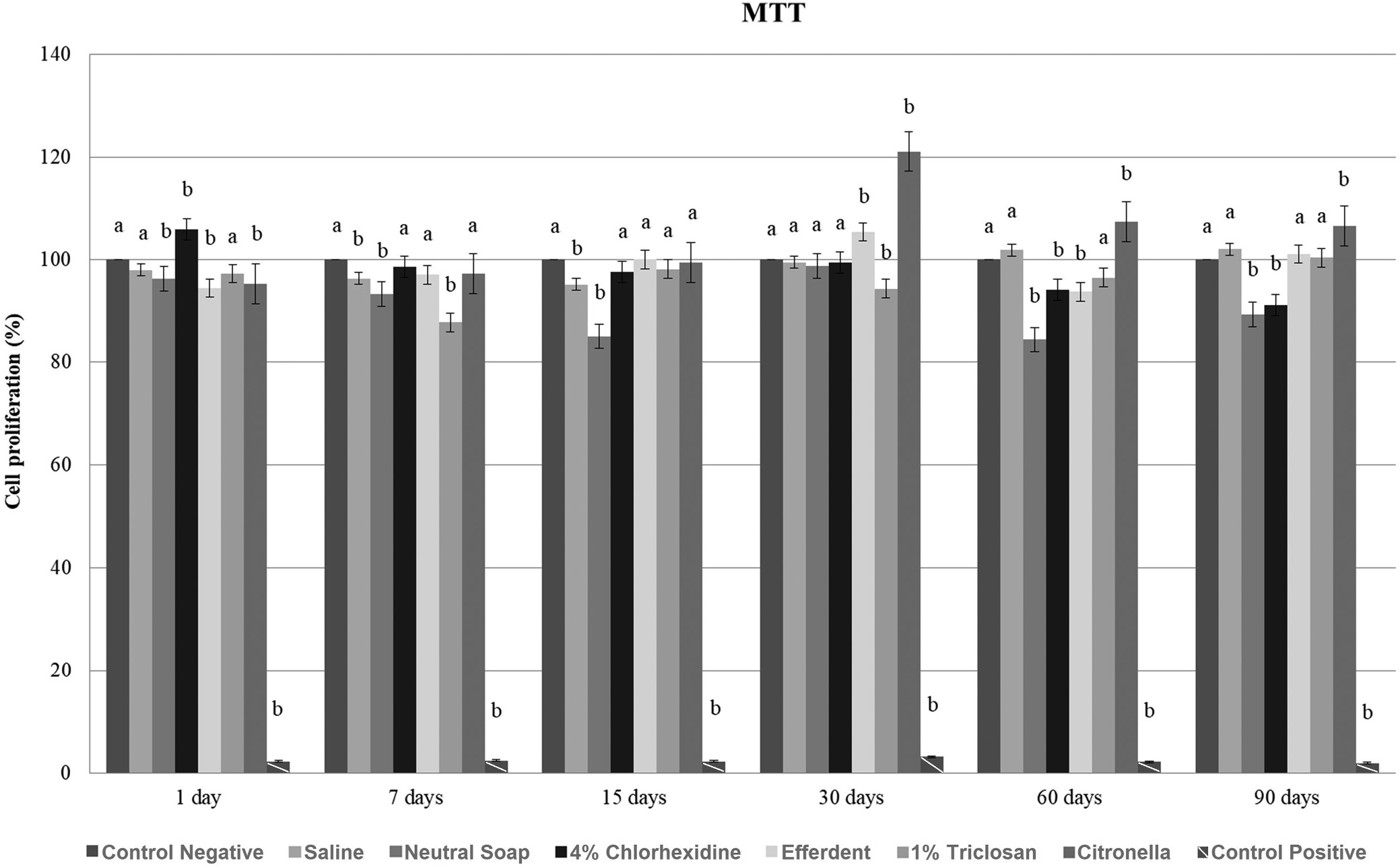
After 7 and 15 days, there was a significant reduction in cell proliferation in the saline group, while in the neutral soap group, such reduction was observed after 1, 7, 15, 60, and 90 days. After 1 day, there was a significant increase in cell proliferation in the chlorhexidine group, followed by a significant reduction after 60 and 90 days. After 1 and 60 days, there was a significant reduction in cell proliferation in the effervescent group, and a significant increase after 30 days. In the triclosan group, after 7 and 30 days, there was a significant reduction in cell proliferation. After 1 day, there was a significant reduction in cell proliferation in the citronella group, followed by a significant increase after 30, 60, and 90 days.
These results are presented in Figure 1.
NR (disinfectant compared to control)
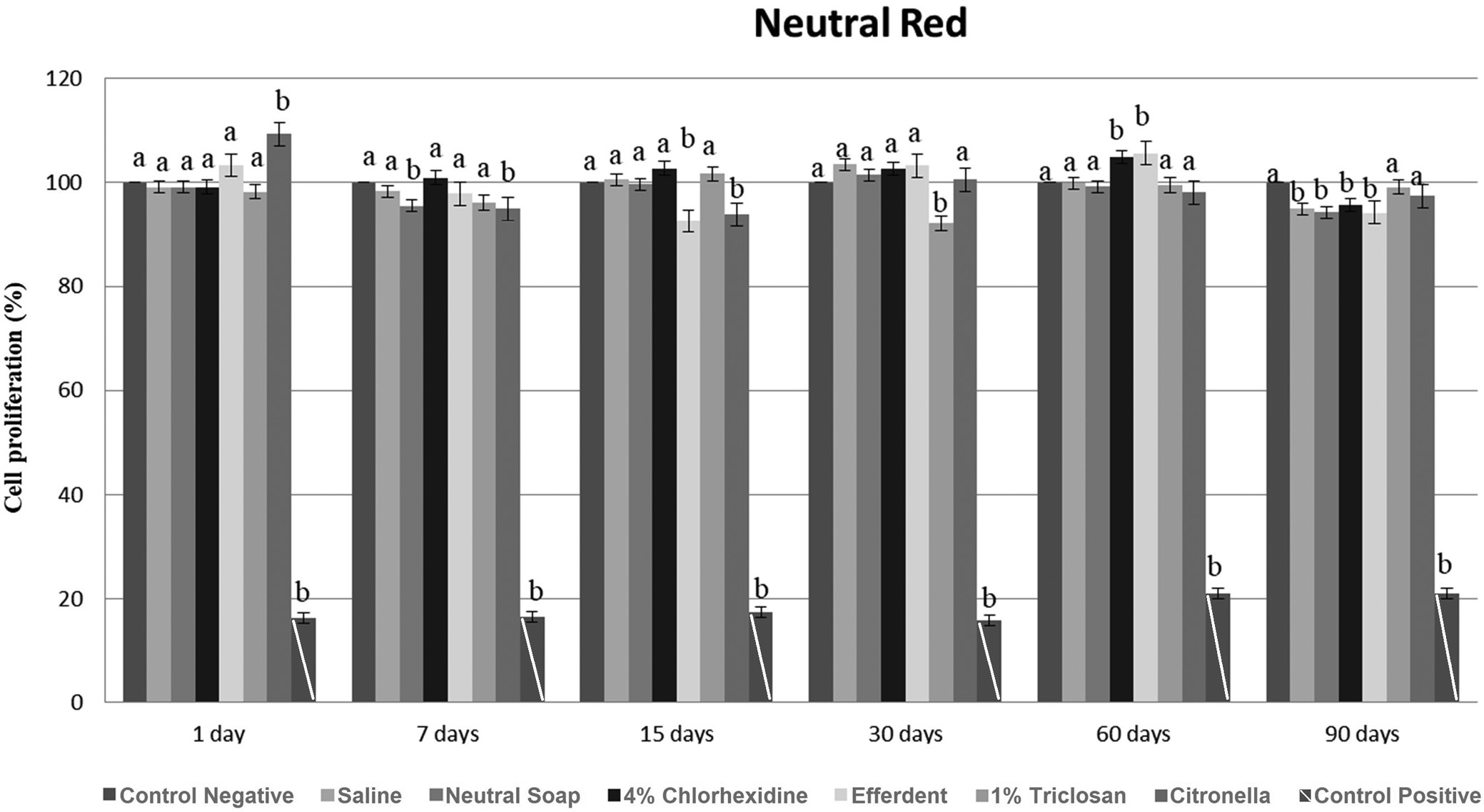
After 90 days, there was a significant reduction in cell proliferation in the saline group. In the neutral soap group, there was a significant reduction in cell proliferation after 7 and 90 days. After 60 days, there was a significant increase in cell proliferation in the chlorhexidine group, followed by a significant reduction in cell proliferation after 90 days. In the effervescent group, there was a significant reduction in cell proliferation after 15 and 90 days, and a significant increase after 60 days. After 30 days, there was a significant reduction in cell proliferation in the triclosan group. There was a significant increase in cell proliferation in the citronella group after 1 day, followed by a significant decrease after 7 and 15 days.
These results are presented in Figure 2.
COL IV
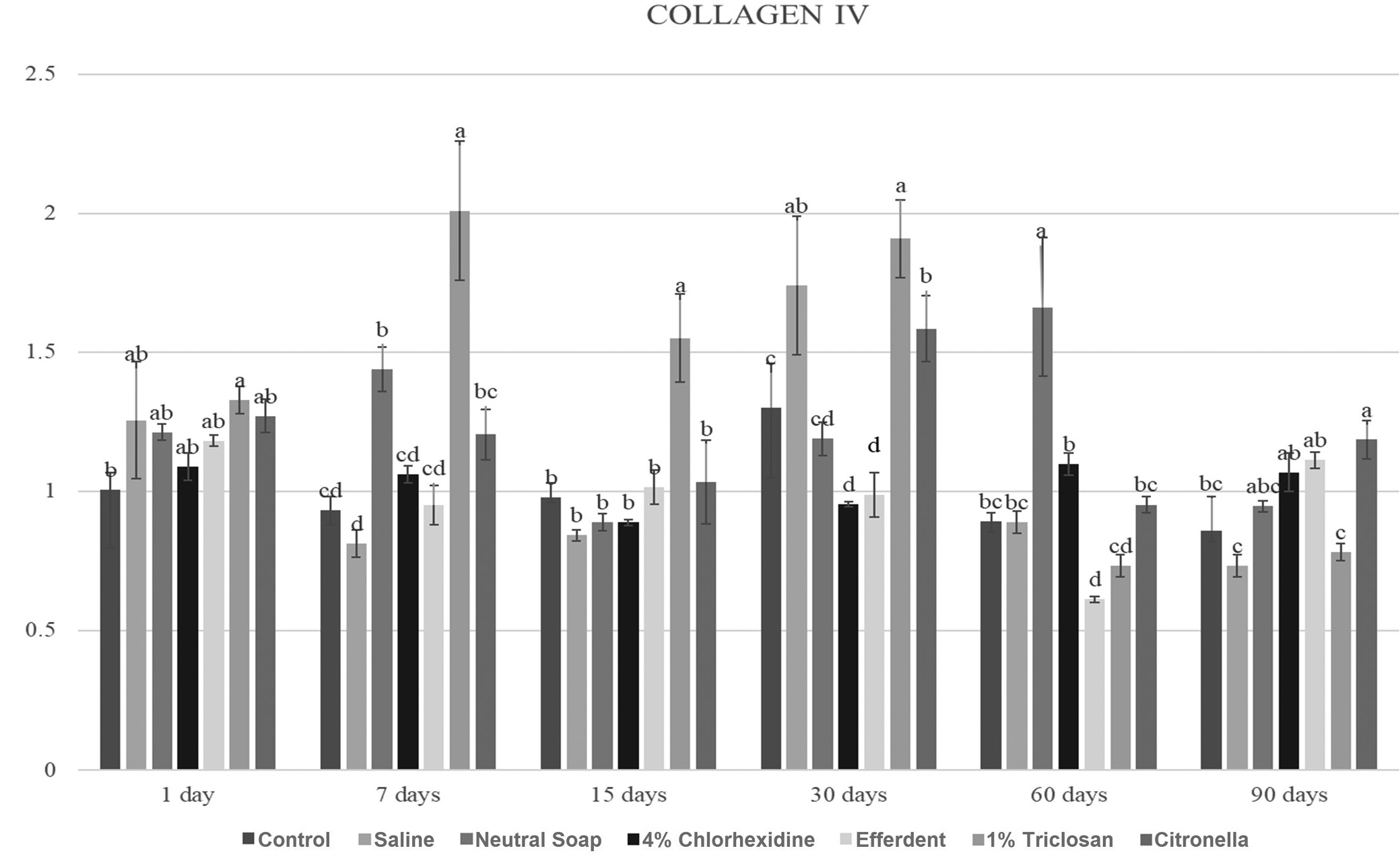
Due to the large number of comparisons, only the most important are reported below. After 7 days, the triclosan group showed a significantly higher COL IV gene expression level compared to the other groups. After 15 days, the triclosan group showed a significantly higher COL IV gene expression level than the other groups. After 30 days, the saline, triclosan and citronella groups showed significantly higher COL IV gene expression levels compared to the other groups. After 60 days, the neutral soap group showed a significantly higher COL IV gene expression level compared to the other groups.
These results are presented in Figure 3.
TGF-β
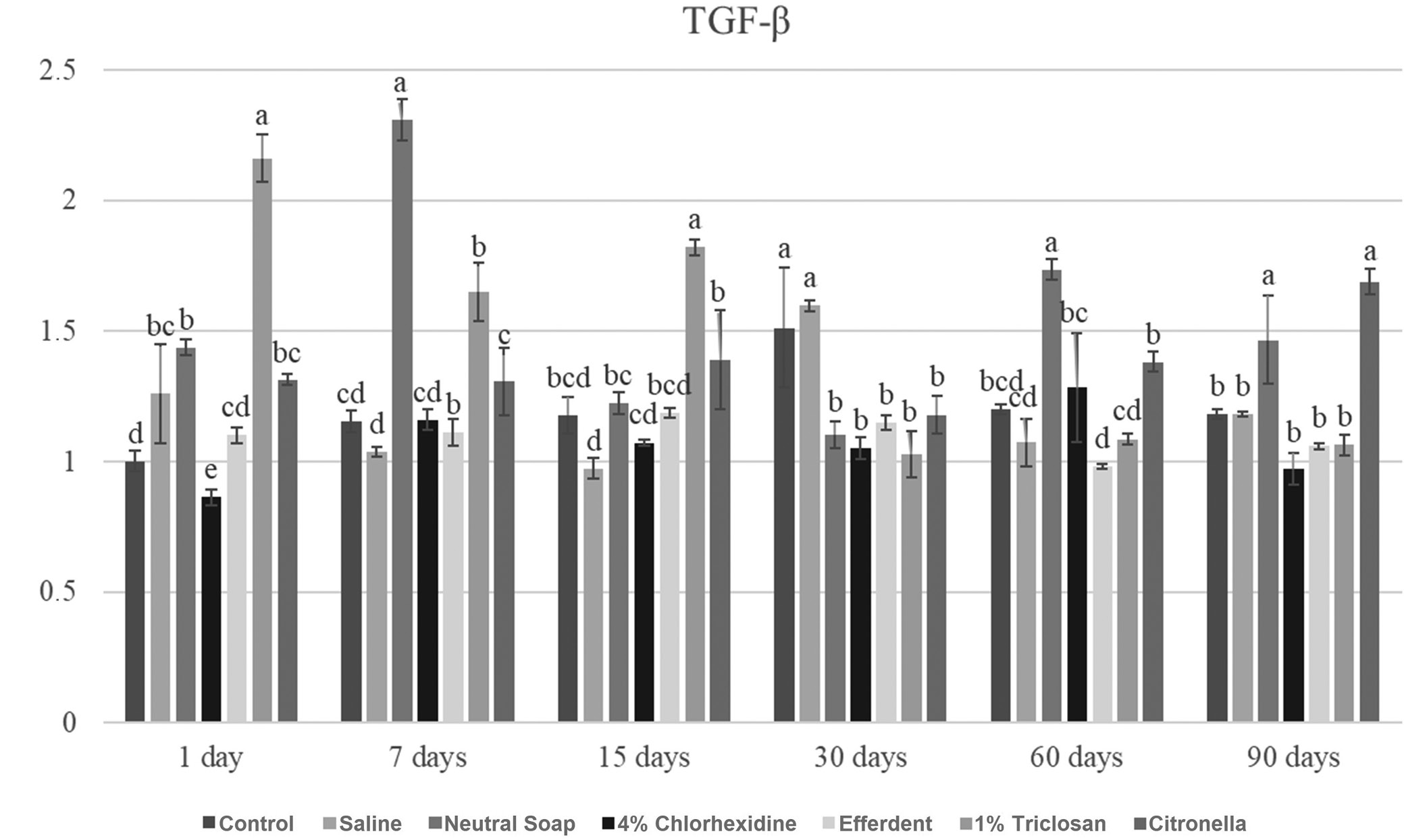
Due to the large number of comparisons, only the most important are reported below. After 1 day, the triclosan group showed a significantly higher TGF-β gene expression level compared to the other groups. After 7 days, the neutral soap group showed a significantly higher TGF-β gene expression level compared to the other groups. After 15 days, the triclosan group showed a significantly higher TGF-β gene expression level compared to the other groups. After 30 days, the control and saline groups showed significantly higher TGF-β gene expression levels compared to the other groups. After 60 days, the neutral soap group showed a significantly higher TGF-β gene expression level compared to the other groups. After 90 days, the neutral soap and citronella groups showed significantly higher TGF-β gene expression levels compared to the other groups.
These results are presented in Figure 4.
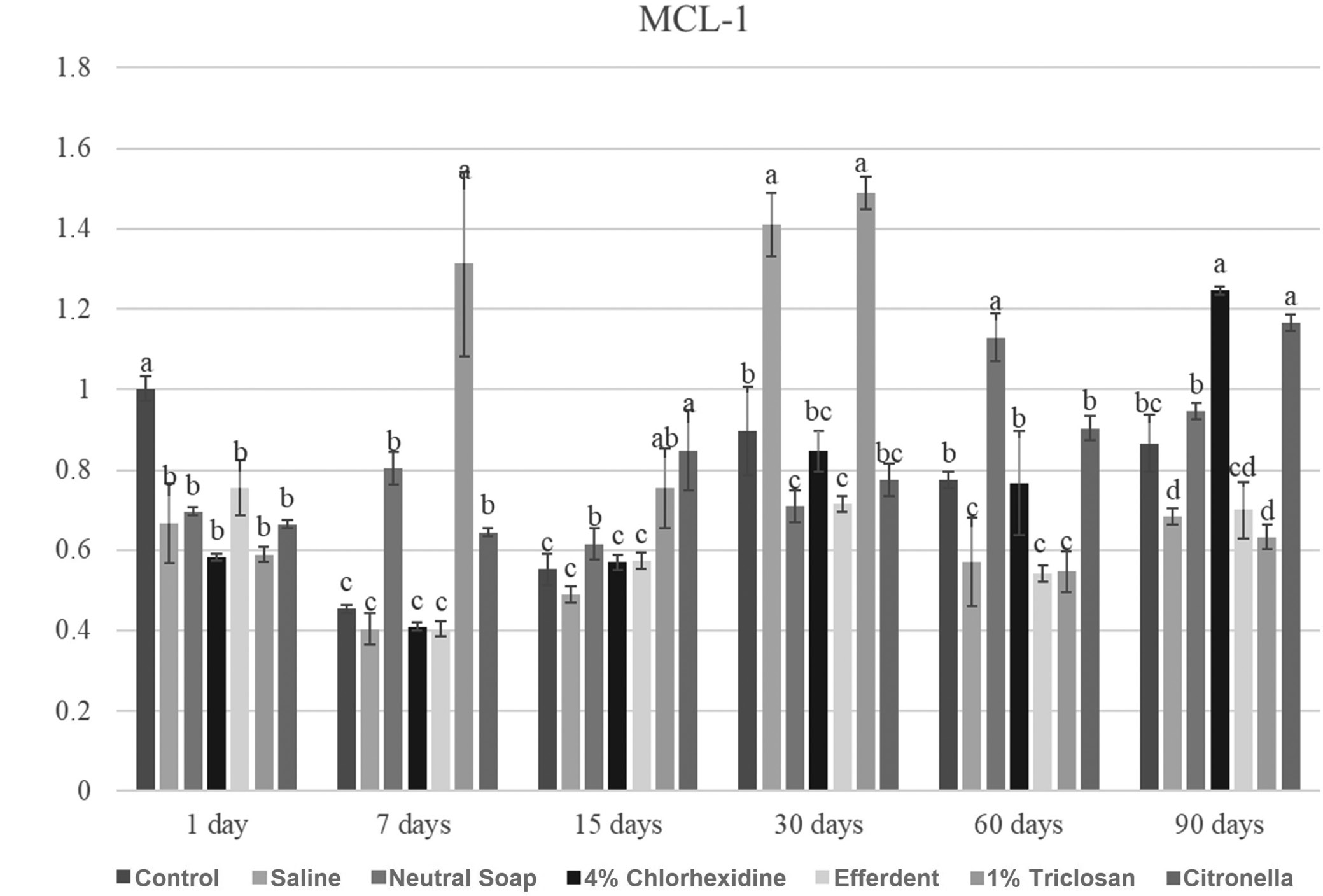
MCL-1
Due to the large number of comparisons, only the most important are reported below. After 1 day, the control group showed a significantly higher MCL-1 gene expression level compared to the other groups. After 7 days, the triclosan group showed a significantly higher MCL-1 gene expression level compared to the other groups. After 15 days, the citronella group showed a significantly higher Myeloid cell leukemia sequence 1 (MCL-1) gene expression level compared to the other groups, except when compared to the triclosan group. After 30 days, the saline and triclosan groups showed significantly higher MCL-1 gene expression levels compared to the other groups. After 60 days, the neutral soap group showed a significantly higher MCL-1 gene expression level compared to the other groups. After 90 days, the chlorhexidine and citronella groups showed significantly higher MCL-1 gene expression levels compared to the other groups.
These results are presented in Figure 5.
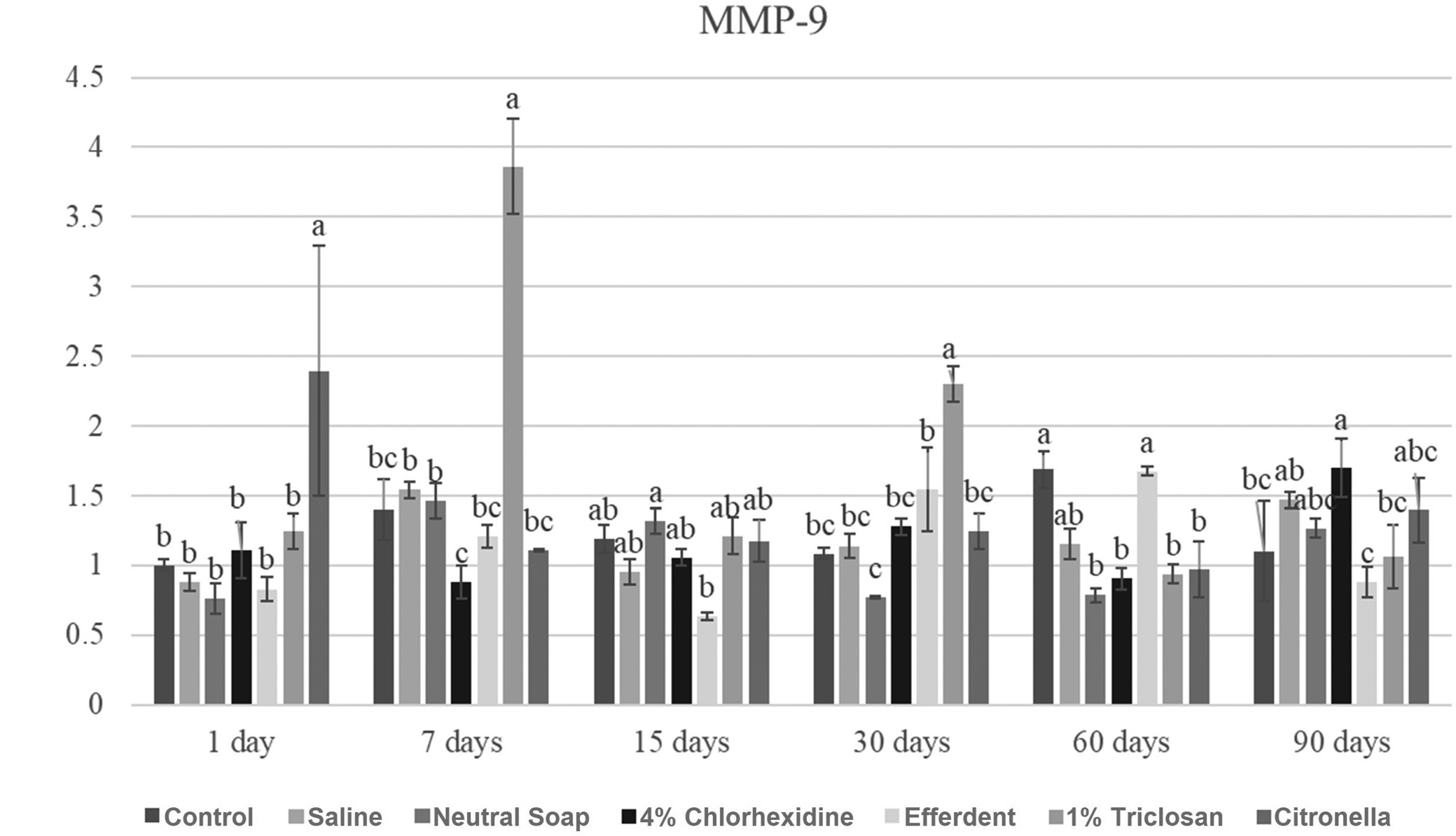
MMP-9
Due to the large number of comparisons, only the most important are reported below. After 1 day, the citronella group showed a significantly higher MMP-9 gene expression level compared to the other groups. After 7 days, the triclosan group showed a significantly higher MMP-9 gene expression level compared to the other groups. After 30 days, the triclosan group showed a significantly higher MMP-9 gene expression level compared to the other groups. After 60 days, the control and Efferdent groups showed significantly higher MMP-9 gene expression levels compared to the other groups, except when compared to the saline group.
These results are presented in Figure 6.
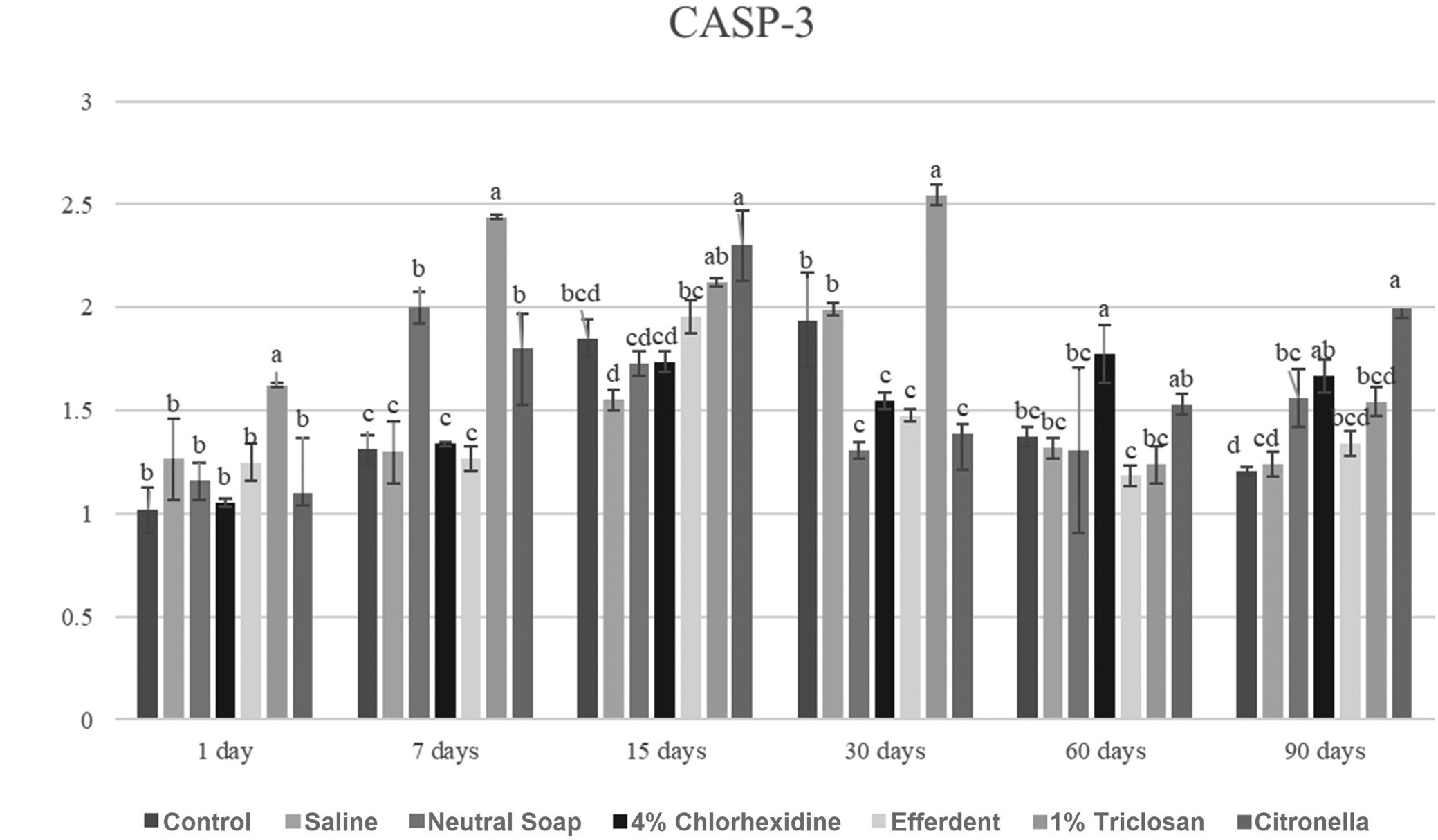
CASP-3
Due to the large number of comparisons, only the most important are reported below. After 1 day, the triclosan group showed a significantly higher CASP-3 gene expression level compared to the other groups. After 7 days, the triclosan group showed a significantly higher CASP-3 gene expression level compared to the other groups. After 15 days, the citronella group showed a significantly higher CASP-3 gene expression level compared to the other groups, except when compared to the triclosan group. After 30 days, the triclosan group showed a significantly higher CASP-3 gene expression level compared to the other groups. After 60 days, the chlorhexidine group showed a significantly higher CASP-3 gene expression level compared to the other groups, except when compared to the citronella group. After 90 days, the citronella group showed a significantly higher CASP-3 gene expression level compared to the other groups, except when compared to the chlorhexidine group.
These results are presented in Figure 7.
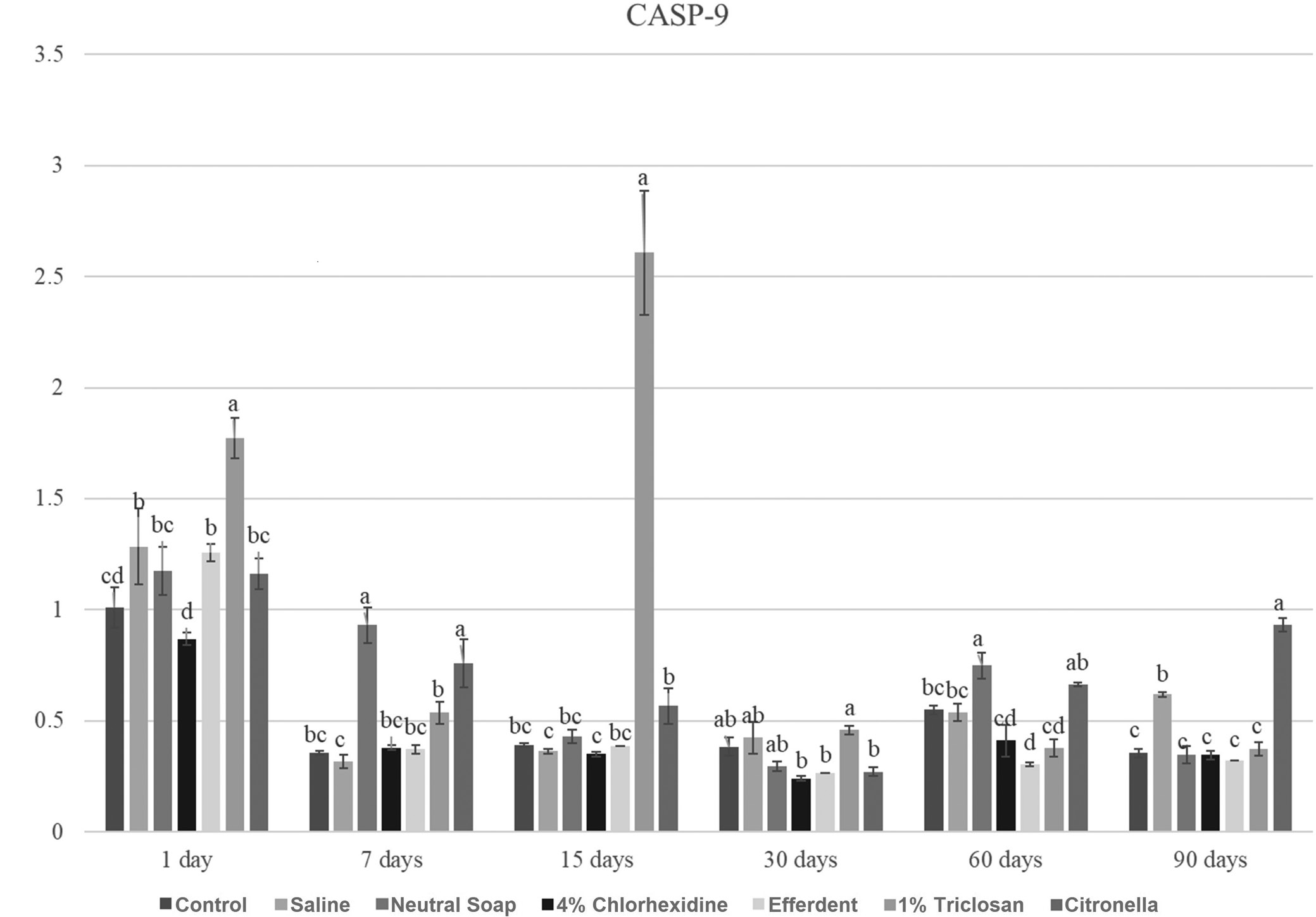
CASP-9
Due to the large number of comparisons, only the most important are reported below. After 1 day, the triclosan group showed a significantly higher CASP-9 gene expression level compared to the other groups. After 7 days, the neutral soap and citronella groups showed significantly higher CASP-9 gene expression levels compared to the other groups. After 15 days, the triclosan group showed a significantly higher CASP-9 gene expression level compared to the other groups. After 60 days, the neutral soap group showed a significantly higher CASP-9 gene expression level compared to the other groups, except when compared to the citronella group. After 90 days, the citronella group showed a significantly higher CASP-9 gene expression level compared to the other groups.
These results are presented in Figure 8.
Discussion
Based on the MTT and NR tests (Figure 1, Figure 2), although statistically significant differences were observed at each time point between the cleaning agents and the control group, all values were greater than 84%. Based on ISO 10993-5, a substance evaluated (in vitro) can be classified as non-cytotoxic (cell proliferation greater than 75%), slightly cytotoxic (cell proliferation between 50 and 75%), moderately cytotoxic (cell proliferation between 25 and 50%), and highly cytotoxic (cell proliferation less than 25%).13 Thus, all cleaning agents were classified as non-cytotoxic, regardless of the evaluated disinfection period.
Based on the statistical evaluation (Figure 1, Figure 2), in most cases, when there was a significant difference between the disinfectant and the control group, there was a significant reduction in cell proliferation generated by the disinfectant. This was probably due to liquid sorption processes and the solubility of PMMA.7 Thus, this material may have absorbed chemical agents from the disinfectant used, which remained inside, making it impossible to remove these absorbed substances by rinsing. Later, probably during cell proliferation, due to the solubility process, these chemical substances were released on the surface of the specimen, resulting in decreased cell proliferation. However, these significant reductions in cell proliferation can be considered clinically acceptable based on previously reported ISO 10993-5 criteria.13 Furthermore, the significant increase in cell proliferation after disinfectant use can be explained by the failure of PMMA to absorb the disinfectant, causing the rinse to completely remove it from the surface of the material (Figure 1, Figure 2). Thus, cell proliferation was not negatively influenced by the disinfectant and increased.
Andreotti et al. observed that 4% chlorhexidine, 1% triclosan and Efferdent significantly reduced the amount of microorganism colonies (Staphylococcus aureus and Staphylococcus epidermidis) compared to citronella oil and neutral soap.5 They also found no significant differences between the 4% chlorhexidine, 1% triclosan and Efferdent groups based on microbial reduction of Staphylococcus aureus and Staphylococcus epidermidis.5 Also in the study by Andreotti et al., it is noteworthy that neutral soap was significantly more effective in reducing Staphylococcus aureus than citronella oil, and had the same effect against Staphylococcus epidermidis as citronella oil.5 Thus, as all disinfectants tested in this study were classified as non-cytotoxic – 4% chlorhexidine, 1% triclosan and Efferdent are the disinfectants indicated for cleaning ocular prostheses, as they combine high antimicrobial efficiency and absence of cytotoxicity against human conjunctival cells.
Regarding the expression of COL-IV, TGF-β, MCL-1, MMP-9, CASP-3, and CASP-9, the triclosan group was the one that stood out the most among the groups (Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8). This group presented, on several occasions, significantly higher values than the other groups (Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8), which were very expressive. Higher levels of TGF-β expression stimulate the synthesis of COL IV. On the other hand, the MMP-9 expression acts to degrade COL IV.14, 15 Therefore, a balance between the synthesis (related to TGF-β) and breakage of collagen (related to MMP-9) is clinically important.1, 3, 4 If a greater expression of TGF-β is not compensated by a greater expression of MMP-9, a fibrous reaction during the tissue repair process may occur, compromising the use of the ocular prosthesis. On the other hand, if there is an increase in the expression of MMP-9, not compensated by the increase in the expression of TGF-β, severe tissue damage may occur, also affecting the patient’s rehabilitation.16 It is noteworthy that COL IV, produced by epithelial cells, is essential in the composition of their basement membrane extracellular matrix.4 In turn, MCL-1 (anti-apoptotic protein) promotes cell survival,1, 12 while CASP-3 and -9 are proteases involved in apoptosis,1, 11 and their activation (CASP-3 and -9) induces an increase in reactive oxygen species (ROS).1, 17 The balance of these markers is also important for the health of the anophthalmic socket tissue. Thus, the ocular prosthesis and its cleaning agent must maintain tissue health, preventing the formation of fibrosis, unbalanced cell death and inflammatory reactions. New studies should be carried out using the disinfectants described in this study in different concentrations, in addition to other disinfectants for cleaning ocular prostheses.
Conclusions
All reductions in cell proliferation generated by the disinfectants were clinically acceptable. All disinfectants tested in this study were found to be non-cytotoxic to human conjunctival cells.