Abstract
Background. Temporary prosthesis protects the oral tissues, in addition to providing aesthetic look and masticatory function until a definitive prosthesis is manufactured.
Objectives. To evaluate the effect of glaze and 0.12% chlorhexidine (CHX) on the physical and mechanical properties of bis-acryl, and to evaluate the antimicrobial efficacy of CHX.
Materials and methods. Eighty specimens of bis-acryl resin were made. Over 40 of them the glaze was applied. One specimen with and 1 specimen without glaze were placed in niches of an appliance manufactured for each patient. Each of the 20 volunteers received 2 devices. Initially, the volunteers used one device and treated it with sucrose for 7 days (control), and later they used the other device and treated it with sucrose and CHX for 7 days (test). Color, microhardness, roughness, surface energy, and insoluble extracellular polysaccharides (EPS) tests were performed. All results were submitted to the Tukey’s test, with the exception of the EPS results, which were submitted to the Student’s t test.
Results. The ΔE00 of the unglazed control group was significantly higher than that of the unglazed test group. In all groups, a significant decrease in microhardness occurred over time. At both times, the glaze significantly increased the microhardness of the specimens (in all the glazed groups). At the final time, the test glaze group showed significantly higher microhardness compared with the control glaze group. Roughness in the groups without glaze increased significantly with CHX treatment over time. At both times, the glaze generated a significant reduction in roughness in the control and test groups. There was a significant reduction in surface energy over time in all groups. In most comparisons, the glazed groups showed significantly higher surface energy values compared with the unglazed control group. At the final time point, the unglazed test group showed a significantly higher surface energy value than the unglazed control group; and the glazed test group showed a significantly higher surface energy value compared with the glazed control group. The resins that received CHX had a significantly lower amount of biofilm.
Conclusions. Color values were clinically acceptable in all tested groups. At both time points, the roughness values were clinically acceptable only in the glazed groups. Glaze increased the microhardness of the specimens. Microhardness and surface energy were reduced over time in all groups. Chlorhexidine can help prevent microhardness degradation. Glaze and CHX can increase surface energy. Chlorhexidine reduced the amount of bacterial biofilm.
Keywords: bacterial adhesion, surface property, temporary dental restorations, dental biofilm
Background
Temporary restorations (or provisional prostheses) can be used for long periods of time to enable the evaluation the results of periodontal and endodontic treatment, and during the phases of making a porcelain crown.1, 2 They have the function of protecting periodontal and dental tissues, in addition to restoring chewing, aesthetics and, in many cases, phonetics.1 Materials such as acrylic resins and bis-acryl resins can be used to manufacture temporary restorations.1, 2, 3 In addition, it is worth mentioning that bis-acryl resins have excellent aesthetics, a relevant factor for patients.
Mouthwashes are widely used in the prevention and control of caries and periodontal diseases, even without a prescription from a dentist.3, 4 Chlorhexidine (CHX) is a widely used mouthwash due to its antimicrobial action.3, 5 However, it has the potential to stain restorations, in addition to affecting other factors such as microhardness, roughness and surface free energy.3, 6
Color stability is the property of a material to retain its color for a period of time in a given environment.7 The color change can be influenced by the surface degradation of the polymeric material, as the degraded areas can serve as deposits of pigments present in various substances, such as CHX.3, 7, 8 Adequate microhardness is also important because it is related to the wear resistance of the material, which in turn is important for preserving its surface integrity over time.7 Regarding the surface roughness of a material, it is always recommended that it be as small as possible to avoid microbial adhesion.1, 9 A rough surface can facilitate the adhesion of the microbial biofilm due to a larger contact surface.3 In addition to roughness, surface energy has an considerable impact on bacterial adhesion, as a material with high surface energy attracts more bacteria to its surface than one with low surface energy.1, 10 Moreover, surface energy is related to the material’s ability to repel or attract water (thus, the higher the surface energy value of a material, the more water it will attract).3 It is worth mentioning that a material that easily attracts water can also absorb it, and this can cause degradation of such material over time.3
In the oral cavity, most bacteria can only survive if they adhere to hard surfaces.1 To reduce biofilm retention on the temporary restoration, it must be properly ground and polished before installing it on the tooth. Mechanical polishing materials (e.g., abrasive burs with different grits) are used to increase the smoothness of polymer surfaces, thus decreasing bacterial adhesion on them.7 It is noteworthy that liquid polishing with light curing sealants is also available to obtain smooth surface of a provisional restoration.7
There is a lack of studies that evaluate the alteration of the properties of bis-acryl resins in situ with and without glaze in association with the use of antimicrobial solutions. Therefore, the objective of this research was to evaluate the influence of glaze and 0.12% CHX on the color, microhardness, roughness, and surface energy of the bis-acryl resin. The 2nd objective was to evaluate the antimicrobial efficacy of 0.12% CHX. The null hypotheses were that 1) the analyzed properties would not be altered by the use of 0.12% CHX and glaze application, and that 2) CHX would not have anti-biofilm capacity.
Materials and methods
This study was approved by the Ethics Committee for Research with Human Beings of the Araçatuba Dental School, São Paulo State University, Brazil (process No. 71186117-0-0000-5420). Participants received and signed an informed consent form. This study also followed the guidelines of the Declaration of Helsinki.
Twenty volunteers aged between 18 and 25 years were included in this study.3 Inclusion criteria were: good oral health; good oral hygiene; and absence of gingivitis, periodontitis, carious lesions, systemic diseases, and diseases in the salivary glands.3 In addition, participants who wore any type of dental prosthesis, or orthodontic appliances, or used medications that may alter salivary flow, were excluded.3
Eighty specimens (10 mm in diameter, 2 mm in thickness)3 were made using bis-acryl resin (Protemp 4; 3M ESPE, Two Harbors, USA). The preparation of the specimens followed the manufacturer’s recommendations. Protemp 4 is composed of an organic resin system (dimethacrylate polymer and bis-GMA) and inorganic fillers (zirconia silica, fumed silica, silane, and pigments).
The making of the specimens was carried out in metallic matrix according to the method described by Commar et al.3 After polymerization, the surfaces of all specimens (n = 80) were polished. This polishing was performed in a semiautomatic polishing machine at a speed of 300 rpm under constant irrigation (Ecomet 300PRO; Buhler, Lake Bluff, USA). In this equipment, 2 sandpapers were used to polish the specimens (400 and 800 grit sandpaper; Buehler).3, 7 Each specimen had its thickness measured using a digital caliper (500–171-20B; Mitutoyo, Tokyo, Japan) to establish the correct dimensions (10 mm in diameter, 2 mm in thickness)3.
Half of the specimens (n = 40) were polished with light curing glaze (Megadenta, Radeberg, Germany).3, 7 The application of the glaze was carried out with the aid of a brush (KG Brush; KG Sorensen, São Paulo, Brazil).7 After 20 s, the glaze layer was photoactivated for 180 s (Strobolux; EDG Equipamentos, São Carlos, Brazil).3, 7 Thus, the bis-acryl specimens were divided into 2 groups (without and with glaze).
For each volunteer, 2 palatal appliances were made. One device was for control condition analysis and the other was for test condition analysis. The devices were made from chemically activated acrylic resin (Jet; Clássico, São Paulo, Brazil), and each contained 2 niches in the palatal region with dimensions of 10 × 2 mm.3 For each device, 1 unglazed specimen was placed in one niche and 1 glazed specimen was placed in the other niche.3 The specimens were fixed in these niches with sticky wax (Kota, São Paulo, Brazil), and covered with a plastic mesh, which was positioned at a distance of 1 mm from the specimens.3 These procedures were performed to avoid displacement of the specimens from their position.3
Initially, the subjects used the first device with the specimens (control group) for 7 days and applied, with a dropper, a drop of 30% sucrose solution on the specimens 6 times a day (sucrose diluted in distilled water was made in a compounding pharmacy (Pharmacy Manipullis, Araçatuba, Brazil)).3 After applying the solution, each volunteer waited 5 min before repositioning the device in their oral cavity. During this 5-minute period, the device was not rinsed and excess solution was not removed from the device.3 After using the device for 7 days (control group), each volunteer did not use any other device for another 7 days (“rest period”). Then, each volunteer started to use their other device containing the test group specimens.3 The period of use of this other device (test group) was 7 days.3 A drop of 30% sucrose was dispensed over the specimens 6 times a day (test group).3 One minute after the 3rd and 6th sucrose application, the specimens (test group) received a drop of 0.12% CHX without dye and alcohol (Pharmacy Manipullis).3 Each volunteer then waited 4 min to place the device (test group) in their oral cavity.3
The volunteers received guidance on cleaning the palatal device with a soft brush and specific toothpaste (Colgate Máxima Proteção Anti-cáries; Colgate Brazil, São Paulo, Brazil) 3 times a day.3 Volunteers were also instructed how to remove the device during meals and clean the oral cavity, and regarding specimen surface treatment protocol.3 All these procedures were performed with the palatal device outside the mouth, but with the specimens properly positioned in their niches.
All specimens (control and test) were submitted to color change, microhardness, roughness, and surface free energy tests before and after the in situ period, corresponding to 7 days. After this period, biofilm collection was also performed on test and control specimens for subsequent insoluble extracellular polysaccharides (EPS) analysis. In all analyses, the plastic mesh was removed and the specimens were removed from their niches.
The color stability (ΔE00) test was performed using a spectrophotometer (UV-2450; Shimadzu, Kyoto, Japan).3, 9 The ΔE00 was calculated using the CIEDE2000 system and the following formula:
ΔE00 = {[ΔL′/(KLSL)]2 + [(ΔC′/(KCSC)]2 + [ΔH′/(KHSH)]2 + RT[ΔC′/(KCSC)] x [ΔH′/(KHSH)]}1/2.3, 9
The microhardness (Knoop) of the specimens was measured using a microdurometer (HMV-2T; Shimadzu).3 Three penetrations with a load of 25 g for 10 s were made on the surface of each specimen with 500 μm of distance between each penetration.3 The values were recorded in Knoop hardness number (KHN). The average of the 3 results obtained was calculated.3
Surface roughness was measured in micrometers with a profilometer (Mitutoyo SJ-400; Mitutoyo) using the cutoff of 500 μm for 12 s to obtain average roughness (Ra).3 One reading was taken over the center of the specimen and then 2 more readings were taken to the right and to the left of the initial reading.9 Then, the average of the 3 results obtained was calculated.
The surface free energy was calculated based on the contact angle.3 Contact angle values were obtained using a goniometer (Krüss DSA20E Easy Drop Goniometer; Krüss Optronic GmbH, Hamburg, Germany) employing the Owens–Wendt–Rabel–Kaelble (OWRK) technique.3, 11
For EPS analysis, formed dental biofilm was collected from all specimens using beveled plastic spatulas.12 To carry out this test, 1.0 mol/L of NaOH (0.1 mL/10 mg dry weight of biofilm) was added to the biofilm precipitate. The EPS analysis was performed by the phenol-sulfuric method. Thus, 25 μL of 80% phenol and 125 μL of 95.5% sulfuric acid were added to the biofilm specimens.12
The values obtained in the analysis of color change were evaluated with two-way analysis of variance (ANOVA). The values for other tests were submitted to three-way repeated measures ANOVA. All results were submitted to the Tukey’s test (p < 0.05), with the exception of the EPS results, which were submitted to the Student’s t-test (p < 0.001).
Results
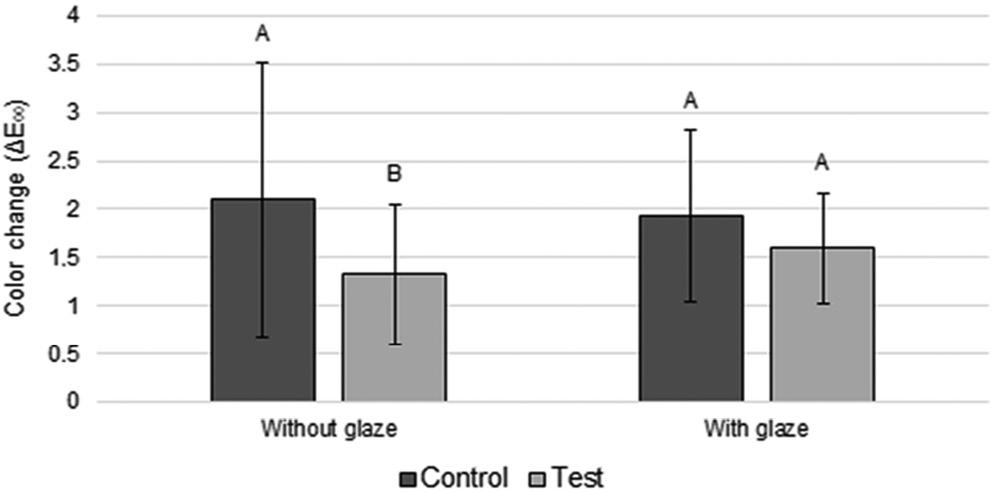
Regarding color evaluation, ANOVA showed that there was a statistical significance for the disinfection factor (p = 0.013) (Table 1). The ΔE00 value in the unglazed control group was significantly higher than in the unglazed test group (Figure 1).
Regarding microhardness, ANOVA showed that the glaze
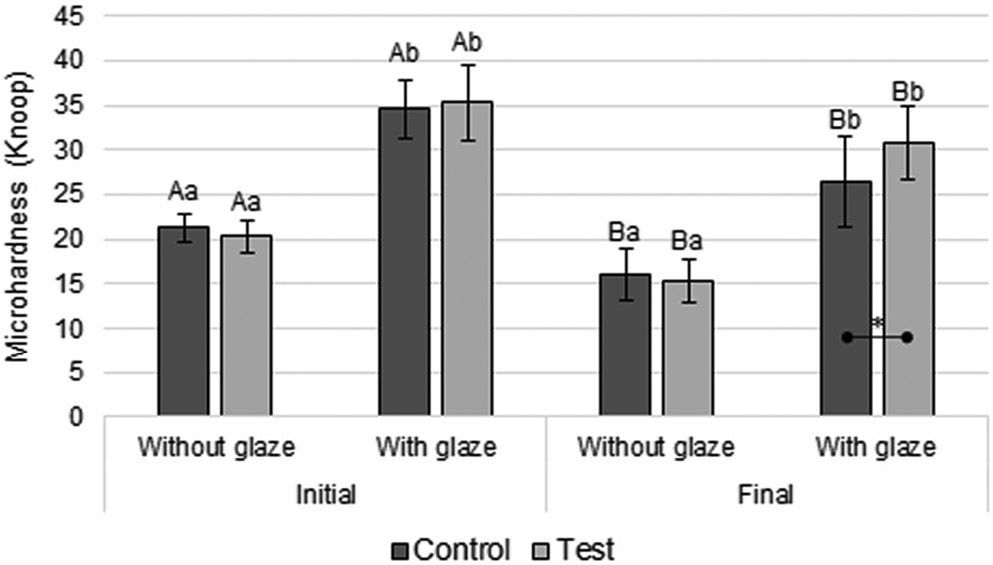
(p < 0.001) and time (p < 0.001) factors, and the interaction between the glaze and disinfection (p = 0.007) and time and disinfection (p = 0.040) were statistically significant (Table 2). In all groups (Figure 2), a statistically significant decrease in microhardness was observed over time. At each time point (initial and final), the groups with the glaze showed a significantly higher microhardness than the groups without the glaze (Figure 2). At the final time point, the test glaze group showed significantly higher microhardness compared with the control glaze group (Figure 2).
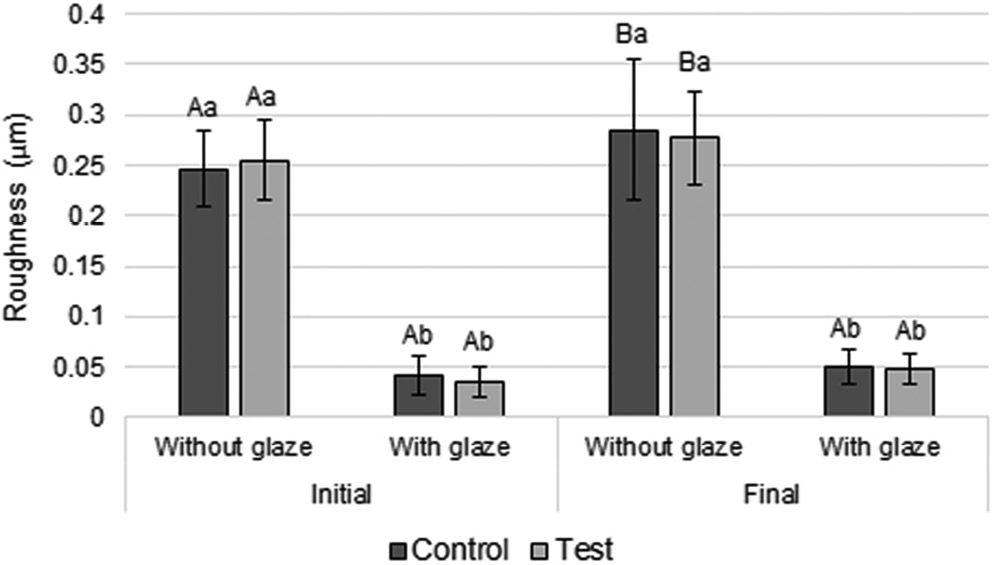
When analyzing surface roughness, ANOVA showed that the interaction between time and glaze factors showed statistical significance (p = 0.033) (Table 3). The roughness values in the groups without glaze (control and test) increased significantly over time (Figure 3). In addition, at both time points, the glazed groups showed significantly lower roughness than the unglazed ones (Figure 3).
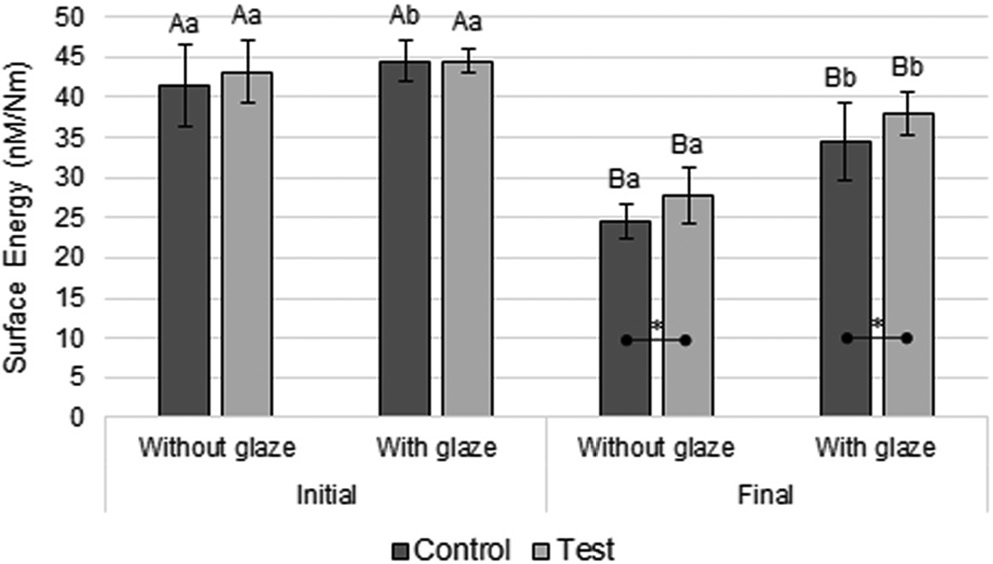
Regarding surface free energy, ANOVA showed that the interaction between time and glaze (p < 0.001) and time and disinfection (p = 0.026) were statistically significant (Table 4). Figure 4 shows that there was a significant reduction in surface energy over time in all groups. At the initial time point, the glazed control group showed significantly higher surface energy value compared with the unglazed control group (Figure 4). At the final time point, the glazed groups showed significantly higher surface energy values than the unglazed ones (Figure 4). In addition, at the final time point, the unglazed test group showed a significantly higher surface energy value than the unglazed control group; and the glazed test group showed a significantly higher surface energy value compared to the glazed control group (Figure 4).
For the EPS analysis, more biofilm volume was required than the glazed and unglazed groups could provide separately, so there was no other possibility but to merge the groups with and without glaze in order to perform the EPS test. Perhaps the time period of 7 days was not enough to form more biofilm. Thus, resins that received 0.12% CHX treatment had a significantly lower amount of biofilm compared with resins that did not receive such treatment.
Discussion
The tested null hypotheses were rejected since the properties of the tested material showed changes due to the use of 0.12% CHX and glaze. In addition, 0.12% CHX showed anti-biofilm capacity on the resin.
Bis-acryl resins have a heterogeneous chemical composition formed by an organic and inorganic matrix and a bonding agent.7 The chemical composition of a material influences the water sorption (absorption and adsorption) and solubility processes that occur with it, causing a material, for example, to absorb more water than another.9 These processes can change the color of the restoration (degradation) due to the exit of water with particles and chemical compounds from the material, as well as the entry of water with particles of different colors and chemical compounds in the material.7, 9 Chlorhexidine has been widely used in oral diseases due to its antimicrobial effect; despite this, it is worth mentioning that it has the potential to stain polymers.6, 13 However, in this study, the unglazed group that received the CHX treatment (without dye) showed significantly lower color change values compared with the unglazed group that did not receive such treatment (Figure 1). This result may be associated with the influence of other extrinsic factors, such as acquired pellicle formation13 and more intensive deposition of microorganisms when CHX was not applied.14 It is worth mentioning that a greater amount of biofilm may result in a greater amount of its toxins that degrade and, consequently, stain the resin.14 Despite this, all groups showed clinically acceptable levels of ΔE00 (ΔE00 ≤ 2.1; Figure 1).3, 9
Microhardness is related to the wear resistance of materials.7 Microhardness significantly decreased in all groups over time (Figure 2), possibly due to water absorption by the resin, which resulted in hydrolytic degradation between the inorganic particles and the organic matrix.7 In addition, at the final time point, the glazed test group exhibited significantly higher microhardness than the glazed control group, probably because 0.12% CHX reduced the amount of biofilm on the surface of the resin used, thus decreasing the amount of toxins generated by the biofilm and, consequently, helping to prevent the degradation of this material. It is worth remembering that the mouthwash used did not contain alcohol in its composition; alcohol would prevent the recognition of the individual effect of CHX on the specimens, as alcohol can degrade the polymer matrix and cause changes in its physical and mechanical properties.3, 4
The groups that received the glaze exhibited a significantly higher microhardness than the groups that did not receive it (Figure 2), presumably because the glaze applied to the surface layer of the specimens had a higher microhardness than the surface of the tested material.7 As the tip of the microdurometer measures microhardness only on the surface of the specimens, it is likely that the reading was taken only on the glaze surface and not on the resin.
Adequate surface smoothness is important for a temporary restorative material because it helps to prevent the accumulation of biofilm on the material, which consequently limits its degradation over time, in addition to preventing gingival inflammation.1, 9, 15 In this study, all specimens that received glaze application showed significantly lower roughness values compared with specimens that did not receive glaze (Figure 3). This may have happened because the glaze has the function of filling microdefects and microcracks present on the surface of the resin, which may remain even after mechanical polishing of the surface of this material.7 In addition, there was a significant increase in roughness over time in all unglazed groups – unlike glazed groups, which did not show a significant increase in roughness values over time (Figure 3). Thus, the glaze likely protected the specimen surfaces from being significantly roughened over time. It is worth noting that, due to the greater microhardness (higher wear resistance) of the glaze layer compared with the bis-acryl resin, the surface smoothness of this layer was not significantly changed over time (Figure 3).
Bacterial adhesion on the surface of a material is facilitated when its surface roughness is greater than 0.2 μm.9, 15 Thus, it is possible to observe that the groups with glaze showed clinically acceptable roughness ≤0.05 μm (Figure 3). On the other hand, all unglazed groups showed clinically unacceptable roughness (>0.2 μm; Figure 3). Therefore, the application of a glaze layer on bis-acryl resin restorations is clinically indicated.
The surface free energy is related to the material’s ability to repel or attract water.3 At both times, the glazed groups had significantly higher surface energy values than the unglazed ones (p < 0.05), except for the test conditions at initial time (p > 0.05; Figure 4), probably because the glaze has a higher surface energy compared with the bis-acryl resin. As previously reported, a material with high surface energy attracts more bacteria to its surface than a material with low surface energy.1, 10 Despite this, at both times, the surface roughness was clinically acceptable only in the groups with glaze, helping to prevent bacterial adhesion to the surface of the material.9, 15 It is worth remembering that the surface energy was significantly reduced over time in all groups (Figure 4). Thus, the application of glaze over the bis-acryl resin is indicated.
At the final time point, the test groups showed significantly higher surface energy values than their respective control groups (Figure 4). This may be related to the positive ionic charge present in CHX,6, 13 which can lead it to the phosphate groups on the surface of the resin, resulting in an increase in the surface energy of the material.3 Thus, although CHX increased the surface energy of the tested material due to its deposition on it, CHX is also bacteriostatic and bactericidal (factors that prevent the increase in the amount of biofilm).
Oral biofilm is the main etiological factor for the development of periodontitis and peri-implantitis.16 Resins that received 0.12% CHX treatment had a significantly lower amount of biofilm compared to resins that did not receive this treatment. This reduction in the amount of biofilm with CHX treatment can be explained, as CHX penetrates the biofilm, altering its formation (bacteriostatic effect) or having a bactericidal effect.16 According to Solderer et al., “as the bacterial cell is negatively charged, the cationic CHX molecule binds to its surface. The integrity of the bacterial cell is thus altered in such a way that CHX can penetrate its inner cell membrane leading to an increase in the permeability of this membrane. This results in leakage phenomena of low molecular weight components from the bacterial cell. At this point, the antimicrobial action is still in the bacteriostatic stage and it can be reversed if the CHX is removed and the bacterial cell is able to recover. If CHX treatment is continued, this may lead to irreversible damage to the bacterial cell (bactericidal effect).”16
The authors of the present study recommend further studies of this nature.
Conclusions
Color values were clinically acceptable in all tested groups. At both time points, the roughness values were clinically acceptable only in the glazed groups. Glaze increased the microhardness of the specimens. Microhardness and surface energy were reduced over time in all groups. Chlorhexidine can help prevent microhardness degradation. Glaze and CHX can increase surface energy. Chlorhexidine reduced the amount of bacterial biofilm.