Abstract
Background. Irvingia gabonensis kernel polymer has gained attention in drug delivery systems because of its compatibility and degradation under natural and physiological conditions.
Objectives. This study aimed to evaluate Irvingia gabonensis polymer as a matrix system for the controlled delivery of ibuprofen in comparison to xanthan gum and hydroxypropylmethylcellulose (HPMC).
Materials and methods. Irvingia gabonensis polymer was extracted using established methods and dried using the oven- and freeze-drying methods. Ibuprofen tablets were prepared by direct compression and the effects of polymer concentration (10–50%), excipients (lactose, microcrystalline cellulose and dicalcium phosphate dihydrate) and polymers (xanthan gum and HPMC) on the mechanical and drug release properties of the tablets were evaluated. Density measurements and the Heckel and Kawakita equations were used to determine the compression properties of the tablets. Friability, crushing strength and the crushing strength–friability ratio (CSFR) were used to evaluate the mechanical properties of the tablets, while dissolution times were used to evaluate drug release from the matrices. The drug release mechanisms were determined by fitting the dissolution data into classic kinetic equations.
Results. Irvingia gabonensis polymer deformed plastically with a fast onset and a high amount of plastic deformation compared with xanthan gum and HPMC. This polymer was directly compressible and formed intact non-disintegrating tablets; the mechanical and dissolution properties of Irvingia gabonensis polymer tablets generally decreased with increasing concentration of ibuprofen. The ranking of dissolution times was xanthan gum > freeze-dried Irvingia gabonensis > HPMC > oven-dried Irvingia gabonensis. The addition of the excipients improved the mechanical properties of the tablets, aided ibuprofen release, and altered the release kinetics, which was largely defined by the Korsmeyer–Peppas model. Increasing the proportion of xanthan gum and HPMC in the matrices resulted in a decreased amount of ibuprofen released after 9 h, with xanthan gum having a greater effect.
Conclusions. Irvingia gabonensis polymer matrices may be effective in the preparation of controlled release tablets, and their right combination with xanthan gum or HPMC could provide a time-independent release for longer durations.
Key words: polymer, tablet, compression properties, Irvingia gabonensis, controlled release
Introduction
Plant polymers have sparked a lot of interest as excipients in recent years due to their abundance, good biocompatibility, non-toxicity and, in some circumstances, superior drug release properties compared with synthetic polymers.1 Because of their natural origins, they are appealing and suitable alternatives to the pharmaceutical excipients. There will always be a need to develop new excipients to meet drug formulation-specific requirements and to provide more effective and less expensive alternatives to conventional excipients. Hydrophilic polymers have been widely used as a directly compressible polymeric matrix for controlled and targeted drug delivery of pharmaceutical formulations. The direct compression method is an economical method for the preparation of matrix tablets due to its simple manufacturing process compared to other controlled release systems.2, 3 Owing to their hydrophilic properties, a variety of natural and modified polymers, including xanthan gum, alginates, guar gum, carrageenan, karaya gum, and khaya gum,1, 2, 4, 5, 6 have been successfully used in the preparation of oral controlled release matrix tablets. A few of these polymer matrices are very effective in offering zero-order time-independent drug release kinetics and, in some cases, they have outperformed established polymers.
The seeds of Irvingia gabonensis (O’Rorke) Bail (Irvingiaceae family), also known as African bush mango or wild mango, has recently gained much interest. Irvingia gabonensis seed contains lipids and polymeric substances; the lipids from its seed are useful as a suppository base,7 tabletting lubricant8 and sustained release agent9; additionally, the mucilage has been used as emulsifying and suspending agent,10 polymer for microbead formulations11 and tablet binder.4 When Irvingia gabonensis was used as a binding agent in metronidazole tablets, they possessed lower mechanical strength and slower drug release properties than standard gelatin binder.4 Recent studies have reported the material and compression properties of Irvingia gabonensis kernel polymer.12 The results showed that this polymer was directly compressible and formed intact non-disintegrating tablets with acceptable crushing strength and friability, comparable with standard polymers.12 Irvingia gabonensis kernel polymer provided controlled release of model drugs when used as binding agent in metronidazole tablets and polymer for the formulation of microbeads, indicating its utility as a controlled release polymer.4, 11 However, the suitability of Irvingia gabonensis polymer as a directly compressible excipient for the formulation of controlled release matrix tablets has not been investigated.
Thus, in the present study, Irvingia gabonensis polymer was evaluated as a directly compressible controlled release excipient in ibuprofen matrix tablets in comparison with xanthan gum and hydroxypropylmethylcellulose (HPMC). The tablet properties and drug release from the matrices were studied, as well as the effects of drug concentration, excipient, polymer type, and polymer concentration on the release kinetics of the matrix formulations. Drug release mechanisms were also investigated to determine the effects of formulation excipients and other parameters on the drug release characteristics of matrix tablets.
Materials and methods
Materials
The materials used in the study included: ibuprofen (BASF AG, Ludwigshafen, Germany), microcrystalline cellulose (MCC; Person Pharmaceuticals Ltd., Backinghenshire, UK), dibasic calcium phosphate anhydrous, dihydrate, dicalcium phosphate (DCP) dihydrate (BDH Chemicals Ltd., Poole, UK), directly compressible lactose (BDH Chemicals Ltd.), xanthan gum (Myprotein Co., Manchester, UK), hydroxypropylmethylcellulose (Ranbaxy Laboratories Ltd., Gurgaon, India), and Irvingia gabonensis mucilage (from Irvingia gabonensis kernel (dika nut), bought on the local market at Okolobiri, Nigeria). The procedure for the extraction of the polymer has been reported elsewhere.4, 12
Preparation of matrix tablets
by direct compression
Ibuprofen–polymer matrix tablets were prepared to contain different concentrations (10% w/w, 20% w/w, 30% w/w, and 50% w/w) of ibuprofen. The drug–polymer blend was mixed in a mixer (VSF 3843C; Forster Equipment Co. Ltd., Leicester, UK) for 10 min. Tablets (500 ±10 mg) were compressed for 30 s at different predetermined loads in a 10.5 mm die, in combination with flat-faced upper and lower punches, using a Carver hydraulic hand press (Model C; Carver Inc., Menomonee Falls, USA). Before compression, the die and flat-faced punches were lubricated with a 1% w/v dispersion of magnesium stearate in acetone. The tablets were then stored in an airtight container over silica gel for 24 h to allow elastic recovery and hardening to occur. Their weight and dimensions were determined, and the relative density of the tablets was calculated.13
The effects of excipient (microcrystalline cellulose, lactose and dicalcium phosphate) and polymers (xanthan gum and HPMC) and drug:Irvingia gabonensis:polymer ratio (1:3:1, 2:7:1, 2:6:2, 2:5:3, 2:4:4, and 2:0:8) were also evaluated.
Compression properties
The compression properties of the polymers were analyzed using Heckel and Kawakita equations.14, 15, 16, 17, 18 The Heckel equation is used to compute the relationship between the relative density of the powder bed during compression (D) and the applied pressure (P), which is expressed as:
ln [1/(1 − D)] = K P + A, (1)
where K is the slope of the linear part, which is inversely proportional to the material’s mean yield pressure (Py); and ln means natural logarithm. The intercept (A) was used to compute the relative density DA using19:
DA = 1 − e−A (2)
The relative density DB, which characterizes the phase of rearrangement at low pressures, may be calculated using:
DB = DA – D0 (3)
The degree of volume reduction (C) in the Kawakita linear model17 is expressed as:
C = (Vo − Vp)/Vo = a b P/(1 + b P), (4)
where Vo is the initial bulk volume and Vp is the bulk volume after compression. Equation 5 can be rewritten as:
P/C = P/a + 1/ab (5)
The constant a defines the minimum porosity of the material before compression, whereas b represents its plasticity. The pressure term Pk is obtained by taking the reciprocal of b.20
Tablet properties
Tablet crushing strength (CS) was determined with a DBK tablet hardness tester (model EHO1; DBK Itruments, Mumbai, India), while the tablet friability (F) was determined with a Thermonik Friability Apparatus (model C-FTA 20; Campbell Electronics, Mumbai, India) for 4 min at 25 rpm (100 revolutions). All tests were done in triplicate.
Disintegration test
The disintegration times of the matrices were determined with a disintegration tester model T-TD20 (Campbell
Electronics) in distilled water at 37 ±0.5°C.
In vitro dissolution studies
The dissolution test was performed using the USP XXIII basket method (model T.DR-6; Kshitij Innovations, Ambala, India), with 900 mL media maintained at 37 ±0.5°C rotated at 50 rpm. To mimic the GI condition, the media contained 0.1 M hydrochloric acid (pH 1.2) for the first 2 h and Sorensen’s phosphate buffer (pH 7.4) for the rest of the experiment. Samples (5 mL) were withdrawn at fixed intervals and replaced with fresh media to maintain a sink condition. The sample was diluted and ibuprofen release was measured using a ultraviolet (UV) spectrophotometer at 222 nm (UV-Visible Spectrophotometer model U.V. Pharmaspec 1700E, 23 OCE; Shimadzu Corp., Kyoto, Japan). All tests were performed in triplicate.
Drug release kinetics
The in vitro drug release data were fitted to zero-order,21, 22, 23 first-order,22, 23 Higuchi,24 Hixson–Crowell,25, 26 and Korsmeyer–Peppas27 kinetic equations in order to study the mechanism(s) of drug release.
The zero-order equation is as follows21, 22, 23:
Q = Q0 + k0t, (6)
where Q is the amount of drug release at time t; k0 is the apparent dissolution rate constant or zero-order release constant; and Q0 is the initial concentration of the drug in the solution resulting from a burst effect.
The first-order equation is as follows22, 23:
ln Q = ln Q0 + k1t, (7)
where k1 is the first order release constant.
The Higuchi equation is as follows24:
Q = kH t1/2, (8)
where kH is the Higuchi release constant.
The Hixson–Crowell equation is as follows25, 26:
Qo1/3 – Q1/3 = kst, (9)
where ks is the constant incorporating the surface/volume ratio.
The Korsmeyer–Peppas equation is as follows27:
Qt/Q = kktn, (10)
where kk is the release rate constant which considers the structural and geometric characteristics of the tablet; and n is the diffusional exponent or release exponent, indicative of the drug release mechanism. A value of n = 0.5 indicates Fickian Diffusion (Higuchi Matrix), 0.5 < n < 1.0 indicates anomalous (non-Fickian) diffusion, n = 1.0 indicates case II transport (zero-order release), and n > 1.0 indicates super case II transport.27
Comparing the correlation coefficient values enabled the identification of the best fit model(s).
Statistical analyses
To compare the effects of the drug concentration, excipients and polymers on the mechanical and drug release properties of the tablets, the analysis of variance (ANOVA) was performed using GraphPad Prism v. 4.0 software (GraphPad Software Inc., San Diego, USA). The Tukey–Kramer multiple comparison post-test was used and p ≤ 0.05 was considered significant.
Results and discussion
Compression properties
of ibuprofen matrix tablets
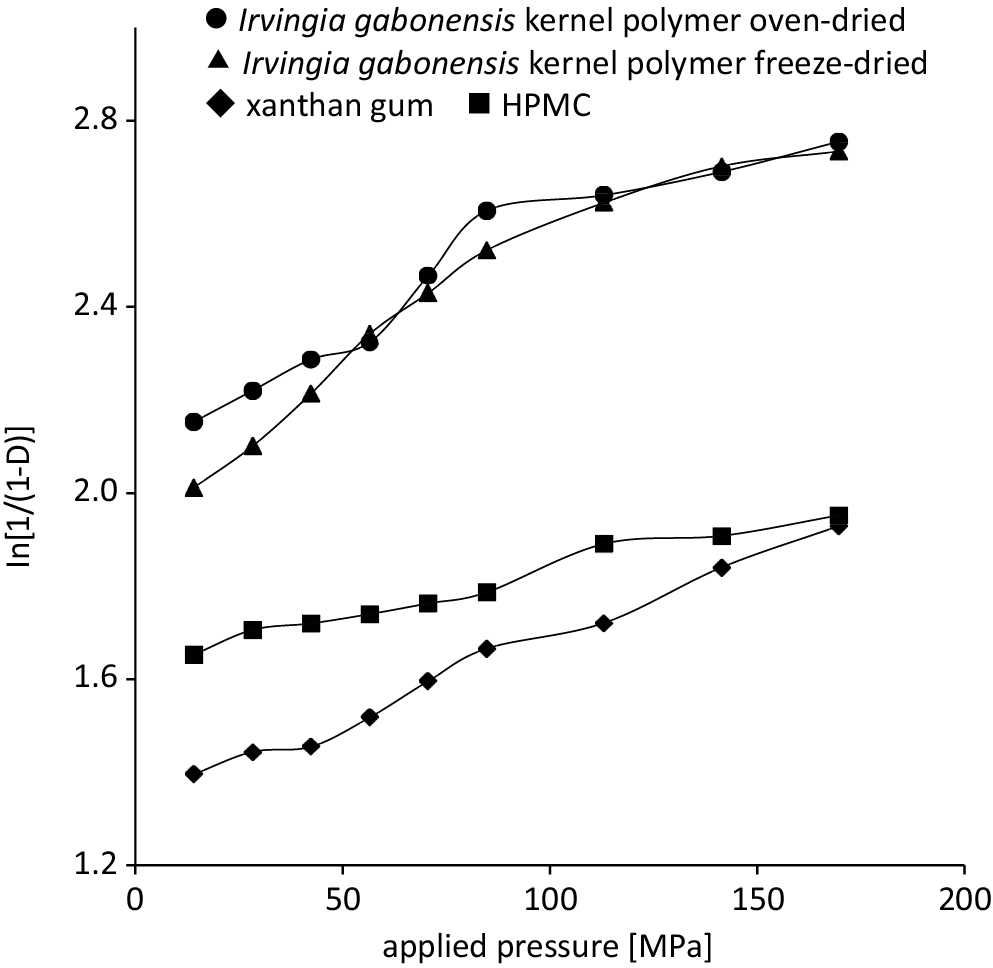
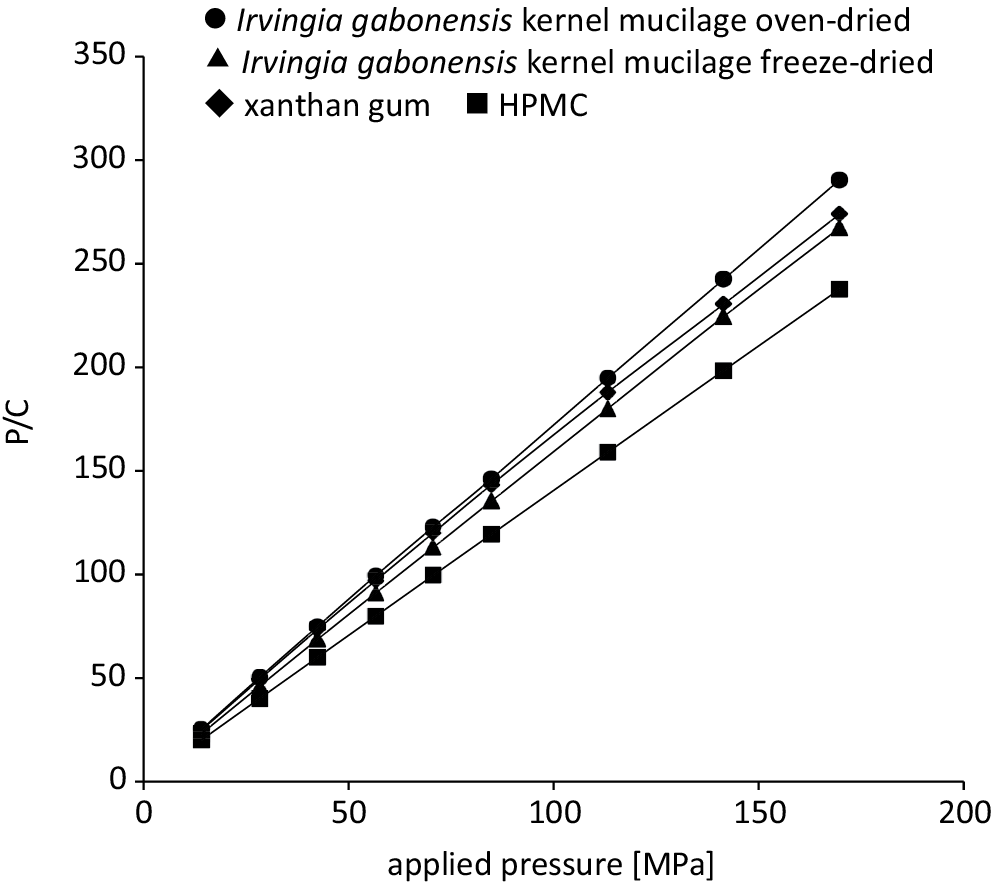
Representative Heckel plots for matrix tablets containing 20% w/w ibuprofen prepared through direct compression are shown in Figure 1. The Heckel plots generally exhibited 2 regions or 2 phases of compression for formulations containing Irvingia gabonensis polymer.28 The mean yield pressure (Py) was calculated from the regions of the plots showing the highest correlation coefficient for linearity, with R ≥ 0.990, generally from 84.82 MPa to 169.69 MPa. Formulations containing HPMC and xanthan gum displayed R ≥ 0.990 at all compression pressures, indicating that the formulations deformed mainly by plastic flow. Representative Kawakita plots for the matrix tablets containing 20% w/w ibuprofen are presented in Figure 2 and demonstrate a linear correlation at all compression pressures with R2 > 0.999. The parameters derived from density measurements and the Heckel and Kawakita plots are presented in Table 1.
It was found that the values of D0 and DI decreased with increasing concentration of ibuprofen. The rankings for D0 and DI were found to be xanthan gum > Irvingia gabonensis > HPMC. There was no significant difference between the density values for freeze-dried and oven-dried Irvingia gabonensis gum, indicating a comparable packing behavior.
In contrast, DA and DB increased as the ibuprofen concentration in the formulations increased. The rankings of DA and DB were found to be freeze-dried Irvingia gabonensis > oven-dried Irvingia gabonensis > HPMC > xanthan gum. There was no significant difference between the densification behaviors of the Irvingia gabonensis mucilage prepared employing both drying methods.
The Pk and Py are pressure parameters that are inverse measures of plasticity.29 The Py relates mainly to the onset of plastic deformation, while Pk relates to the amount of plastic deformation occurring during the compression process.13, 16 The Py values of the formulations increased with an increase in the concentration of ibuprofen whereas Pk generally decreased. This suggested that ibuprofen delayed the onset of plastic deformation but increased the total amount of plastic deformation. Materials that are brittle or easily fragmenting are known to have high Py values, while those that deform plastically or elastically typically exhibit low yield pressure.29, 30, 31 Thus, the addition of a non-polymeric material, such as ibuprofen, reduced the plasticity of the materials but increased the total amount of plastic deformation.29 The rankings for the Py values for the formulations were found to be HPMC > xanthan gum > oven-dried Irvingia gabonensis > freeze-dried Irvingia gabonensis, while the ranking for Pk was found to be xanthan gum > oven-dried Irvingia gabonensis > freeze-dried Irvingia gabonensis > HPMC. Thus, formulations containing Irvingia gabonensis polymer exhibited a faster onset of plastic deformation than HPMC and xanthan gum, and higher amounts of plastic deformation than xanthan gum, but lower deformation than HPMC. Formulations containing HPMC exhibited the slowest onset of plastic deformation while xanthan exhibited the highest amount. High plastic deformation has been related to tablets with high crushing strength and a greater ability to withstand rigorous handling.28
Effect of drug concentration
The mechanical and drug release properties of the ibuprofen matrices are shown in Table 2. It was found that the crushing strength of the matrix tablets decreased with increasing concentration of ibuprofen in the matrix tablets, while the friability increased. The ranking of the crushing strength was found to be HPMC > xanthan gum > freeze-dried > oven-dried Irvingia gabonensis; in contrast, the ranking was reversed for friability. Studies have shown that crushing strength assesses the strength of the tablet while friability tests assess the weakness of the tablet; the greater the crushing strength–friability ratio (CSFR), the stronger the tablet.1, 13, 29 It was determined that tablet crushing strength decreased with an increase in drug concentration, with the following ranks: HPMC > xanthan gum > oven-dried Irvingia gabonensis > freeze-dried Irvingia gabonensis. Polymers are known to undergo plastic deformation, resulting in increased solid bond formation, which increases the strength of the tablet.32, 33 However, the addition of drugs into the polymer reduces the number of solid bonds within the polymer, causing a decrease in tablet strength and an increase in friability. There were statistically significant differences (p < 0.05) in the crushing strength, CSFR and friability values for all the polymers. Freeze-dried Irvingia gabonensis kernel produced tablets with significantly higher (p < 0.05) crushing strength and CSFR, and lower friability values than oven-dried Irvingia gabonensis kernel polymer.
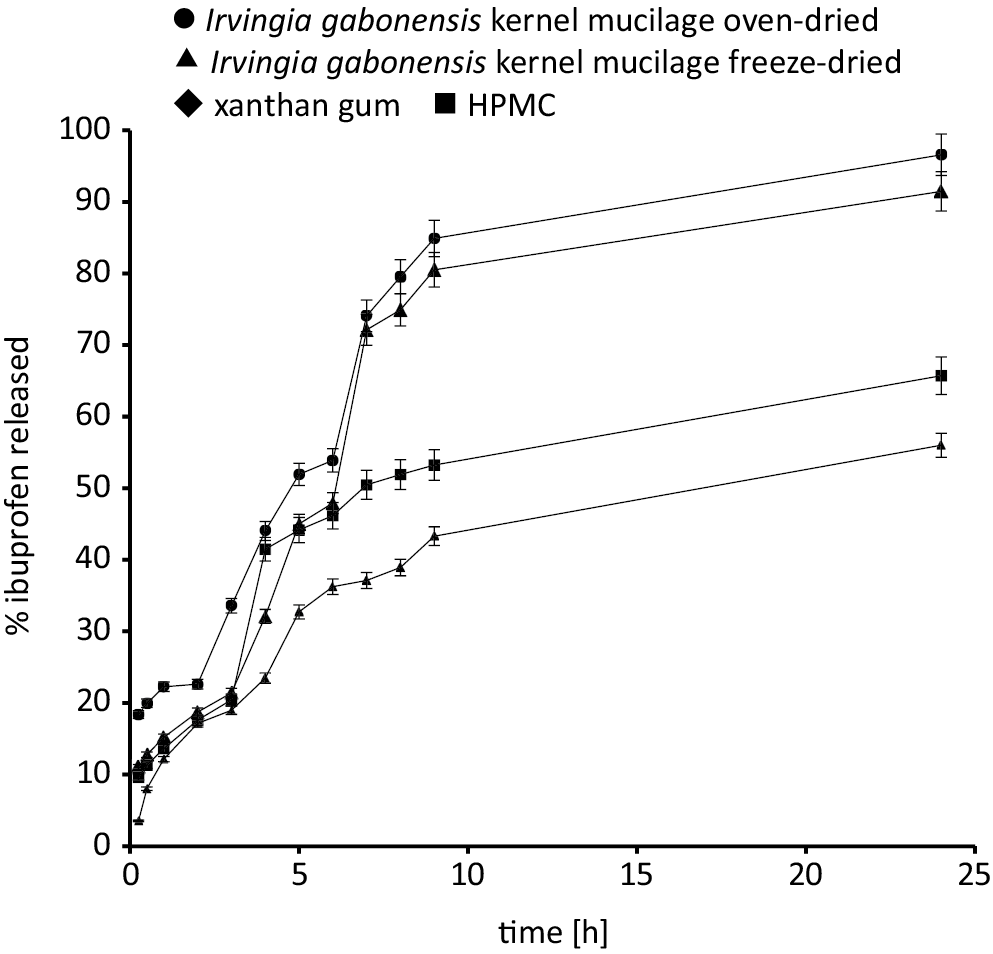
The drug release profiles of ibuprofen tablets containing different drug concentrations are shown in Figure 3, and the time for 25% drug release (t25) is shown in Table 2. It was observed that the dissolution time decreased with an increase in drug concentration in the matrix. The decrease in tablet dissolution times could be attributed to the high concentration of the drug weakening the matrix lattice, which provides a diffusion pathway for matrix erosion/disintegration.34 The ranking of t25 for the formulation was xanthan gum > HPMC > freeze-dried Irvingia gabonensis > oven-dried Irvingia gabonensis. Thus, the formulation containing Irvingia gabonensis polymer exhibited a faster dissolution rate, while xanthan gum showed the slowest dissolution rate.
Drug release kinetics from dosage forms are critical in improving the frequency of administration, bioavailability, patient acceptability, and, in many cases, the occurrence of harmful or toxic consequences.35 The correlation coefficients obtained from the kinetic equations indicating the best fit for each of the models are presented in Table 3. The diffusional exponent or release exponent (n), obtained from the Korsmeyer–Peppas equation, is indicative of the drug release mechanism.27 The drug release parameters derived from the Korsmeyer–Peppas equation for the matrices showed super case II drug release kinetics with n > 1.0. This indicates that drug release from these formulations is controlled by more than one process, usually a combination of diffusion and erosion mechanisms. Drug release from hydrophilic matrices, such as HPMC, has been attributed to the formation of a strong viscous gel when the polymer hydrates in contact with water.32, 36
Effect of excipients
Directly compressible excipients have been used to change the size of drug tablets and to enhance their mechanical characteristics and compression.37 Although the mechanical properties of ibuprofen tablets prepared with Irvingia gabonensis mucilage were acceptable, the tablets became more friable (friability > 1%) as the drug concentration increased.38 Therefore, 3 direct compressible excipients, namely MCC, lactose and dicalcium phosphate (DCP), were added to the matrix tablet formulation in the drug–polymer–excipient at a ratio of 1:3:1. It was found that the mechanical and drug release properties of the tablets (Table 4) with added excipients were significantly (p < 0.001) higher than those without added excipients. The crushing strength and CSFR of the formulations increased, while the friability decreased upon addition of the excipients. There was a significant difference (p < 0.001) in the crushing strength, friability and CSFR of matrix tablets that contained the excipients and of those containing the binary mixtures of ibuprofen–polymer. The ranking of the excipient effect on the crushing strength and CSFR was found to be DCP > lactose > MCC. The ranking was reversed for friability. Comparing all of the polymers, the ranking of excipient effect on crushing strength and CSFR was HPMC > xanthan gum > freeze-dried Irvingia gabonensis > oven-dried Irvingia gabonensis polymer, and it was reversed for friability. Generally, the matrix tablets containing the 3 different excipients had friability values <1%, and thus had enhanced ability to withstand the rigours involved in transport and handling of the formulations.
The addition of the excipients in the matrix tablet formulations facilitated the release of ibuprofen from the tablets. Lactose and MCC had lower t25 values than DCP, although there was no significant difference (p > 0.05) in the dissolution time (t25 and t50 – time for 25% and 50 % drug release, respectively) in both oven- and freeze-dried Irvingia gabonensis kernel matrices. Lactose is a water-soluble excipient that dissolves upon contact with the dissolution media creating a diffusion pathway for the release of the drug. On the other hand, MCC, though water-insoluble, may have largely acted through its disintegrant property, which could facilitate the breakup of the matrix tablet. Collectively, these properties of MCC could increase the dissolution rate and thus cause a faster release of the drug.32
Unlike Irvingia gabonensis kernel polymer, xanthan gum and HPMC standard polymers containing the excipients facilitated a slower release of ibuprofen, as shown by the higher dissolution times. The t25 values of xanthan gum and HPMC matrices that contained the excipients were significantly (p < 0.001) higher than the values for Irvingia gabonensis kernel matrices with the 3 excipients. Hydrophilic polymers such as HPMC have been shown to facilitate prolonged drug release from matrix tablets due to the formation of a strong viscous gel when the polymer hydrates come into contact with an aqueous medium.1, 39
The correlation coefficients from the different dissolution kinetic equations used to determine the drug release kinetics are presented in Table 5. The results showed that drug release from matrix tablets followed the first-order and Korsmeyer kinetic models. The mechanism of drug release depended on the type of polymer and excipient used in the formulation. There appeared to be an interaction between the polymer and excipients, which affected the rate of drug release from the matrix tablets.1 The value of release parameters (n) derived from the Korsmeyer kinetics model depended on the excipient. Formulations containing MCC showed the highest values and DCP the lowest. The release mechanism was generally non-Fickian super case II transport for Irvingia gabonensis matrix tablets with n values greater than 1. On the other hand, the release from xanthan gum and HPMC matrices was found to be anomalous (non-Fickian) diffusion. This non-Fickian release mechanism implies that drug release is controlled by diffusion or a combination of diffusion and macromolecular chain relaxation mechanisms. The above indicates that the nature and type of excipients appeared not to alter the release mechanism of formulations.
Effect of polymers
One method for achieving controlled time-independent release is to use a polymer mixture to achieve sustained release over the desired time. If the 2 polymers are carefully chosen and used in appropriate quantities, it should be possible to create a polymer system with a time-independent release.1, 40 The mechanical and drug release properties of ibuprofen tablets prepared with a polymer mixture of Irvingia gabonensis kernel polymer and standard polymers, xanthan gum and HPMC are shown in Table 6. The results showed that tablets prepared with the drug:Irvingia gabonensis:polymer ratio of 2:7:1 exhibited significantly higher (p < 0.05) mechanical strength than those containing higher concentrations of the standard polymers. Formulations containing HPMC showed higher mechanical strength than those containing xanthan gum. The amount of ibuprofen released after 9 h ranged from 55.34% w/w to 68.96% w/w. Formulations containing HPMC generally showed a slower drug release. There was no significant (p > 0.05) difference in the drug release properties of the formulations.
The parameters obtained from the different drug release kinetic equations (Table 7) showed that the release kinetics from the oven-dried Irvingia gabonensis kernel polymer with xanthan gum and HPMC generally followed the first-order release kinetics, whereas the release of ibuprofen from freeze-dried Irvingia gabonensis kernel polymer matrices containing HPMC and xanthan gum followed the Hixson–Crowell model. The release parameters (n) derived from the Korsmeyer kinetics model indicated that the drug release from the matrix was super case II, suggesting time-independent release kinetics. Thus, the release kinetics of ibuprofen from the matrix tablets prepared with polymer blends appeared independent of the type and concentration of the standard polymer in the polymer blend.
Conclusions
Our results suggest that Irvingia gabonensis polymer is suitable as a directly compressible excipient for the formulation of ibuprofen tablets, comparable to xanthan gum and HPMC. Increasing ibuprofen concentration generally decreased the mechanical and dissolution properties of drug tablets. Inclusion of excipients improved tablet mechanical properties, aided ibuprofen release and altered the release kinetics, which was largely defined by the Korsmeyer–Peppas model. Increasing the proportion of xanthan gum and HPMC in the matrices resulted in a decreased amount of ibuprofen released after 9 h, with xanthan gum having the greatest effect. Irvingia gabonensis polymer could be effective for the preparation of controlled release tablets, and the right combination with xanthan gum or HPMC could provide a time-independent release for longer durations.