Abstract
Background. Burkholderia cepacia adhesion and biofilm formation onto abiotic surfaces is an important feature of clinically relevant isolates. The in vitro biofilm formation of B. cepacia onto coated indwelling urinary catheters (IDCs) with moxifloxacin has not been previously investigated.
Objectives. To examine the ability of B. cepacia to form biofilms on IDCs and the effect of coating IDCs with moxifloxacin on biofilm formation by B. cepacia in vitro.
Materials and methods. The adhesion of B. cepacia to coated and uncoated IDCs with moxifloxacin was evaluated. Pieces of IDCs were coated with moxifloxacin (adsorption method). The spectrophotometric method was used to check moxifloxacin leaching into tubes. Coated and uncoated tubes were incubated with 107 colony forming units (cfu)/mL of B. cepacia. The viable bacterial count was used to count the number of bacteria adhered to coated and uncoated IDC pieces.
Results. A significant adhesion of B. cepacia to uncoated IDC pieces started 15 min after the incubation in a bacterial suspension (107 cfu/mL). A maximum adhesion was observed at 48 h. The pretreatment of IDCs with 100 μg/mL of moxifloxacin produced the best adsorption of antibiotic onto the IDCs. Coating IDC pieces with moxifloxacin significantly reduced the adhesion and biofilm formation of B. cepacia (p < 0.05) at various time intervals (1 h, 4 h and 24 h).
Conclusions. The present study has demonstrated for the first time that coated IDCs with moxifloxacin reduce B. cepacia adhesion and biofilm formation. This finding has opened the door to the production of the new generation IDCs that prevent bacteria from attaching and forming biofilms.
Key words: adhesion, biofilm formation, Burkholderia cepacia, indwelling urinary catheter, moxifloxacin
Background
Burkholderia cepacia is a non-fermenting Gram-negative (NFGN) bacteria responsible for a significant number of hospital infections.1 It has been indicated in chronic nosocomial infections such as septicemia, respiratory tract infections, genitourinary system infections, intracranial infections, endocarditis, gastrointestinal system infections, and surgical site infections.2 It is classified as an important emerging opportunistic pathogen, especially in immunocompromised hosts. Studies have shown that these bacteria maintain their vitality by attaching to any surface and forming biofilms rather than remaining in their planktonic form.3
In addition to having an important role in bacterial adherence and resistance to antibiotics and phagocytosis, biofilms allow bacteria to gain the ability to adapt to the environment by forming a community with new genetic modulations. These help mediate cell–cell and cell–surface interactions critical for biofilm formation and for stabilization of the extracellular exopolysaccharide, produced by bacterial cells.4, 5
Burkholderia cepacia is capable of surviving and multiplying in urine, intravenous fluids and respiratory secretions. Previous studies have shown that B. cepacia causes many clinical problems by attaching to biotic and abiotic surfaces.6, 7 It can be found in humid areas in the hospital such as food, sinks, toilets, floor cleaning cloths, respiratory equipment, dialysis equipment, plastic tubing of catheters, and even disinfectant solutions.8 The experimental work on the adhesion of B. cepacia to the plastic tubing of indwelling urinary catheters (IDCs) is not clearly described in the literature. Subinhibitory concentrations (sub-minimum inhibitory concentrations (sub-MICs)) are defined as concentrations of an antibiotic below the MICs. The sub-MICs of antibiotics can alter the physical and chemical cell–surface features of bacteria, and may hinder the expression of some virulence properties and functionality of bacteria, such as bacterial adherence.9 In recent years, researchers have been interested in identifying alternative treatments that inhibit biofilms and increase the effectiveness of antibacterial drugs, i.e., using heavy metal nanoparticles, enzymes and plant extracts.10
It has been reported that these alternative treatments affect the structure of the biofilm by various mechanisms.10 However, more studies are needed on this subject. Currently, many research projects have been conducted to find new antibacterial targets. Studies focused on preventing the communication between bacteria instead of directly killing them have proposed promising new strategies for the future, but these solutions have yet to be put into practice.11 In this study, we aimed to evaluate the adhesion of B. cepacia to abiotic surfaces, biofilm formation, and the role of subinhibiting doses of moxifloxacin in reducing the ability of this bacterium to form biofilms on IDCs.
Materials and methods
Clinical isolates
In our study, clinical isolates of B. cepacia obtained from the Medical School’s Department of Medical Microbiology in Chandigarh, India, were preserved by lyophilization. To determine biofilm formation, subcultures were prepared every week by inoculating Luria-Bertani Agar (LB; HiMedia, Mumbai, India) medium at 37°C.
IDC tubes
Female 2-way latex foley catheters (Well Lead Medical Co., Ltd., Guangzhou, China) were used in the current study. The catheters are made from natural latex. They have a silicone-coated surface (to reduce allergic reaction), a length of 270 mm, CE and ISO 13485 certificates, and are single-use only. The catheters were opened and the tubes were cut under sterile conditions.
Bacterial adhesion to pieces of IDC tubes
The isolates were grown overnight at 37°C in individual sterile bottles containing trypticase soy water (tryptica soy broth (TSB); HiMedia). A cell pellet was obtained by centrifugation (10,000 g for 5 min at 4°C). The pellet was washed twice with phosphate-buffered saline (PBS, 0.01 M, pH 7.2) and resuspended in sterile PBS (0.01 M, pH 7.2) to adjust the bacterial count to 1×107 colony forming units (cfu)/mL. Several pieces of IDC tubing measuring 1 cm2 were placed in test tubes containing B. cepacia (1×107 cfu/mL) and then incubated at 37°C for different time intervals: 0 min, 15 min, 30 min, 1 h, 2 h, 4 h, 24 h, and 48 h. After the incubation, each piece of tubing was gently washed 3 times with PBS (0.01 M, pH 7.2) solution to remove any unbound bacteria. Each piece was individually scratched in 1 mL of PBS solution. Then, 100 µL were serially diluted and plated on double plates of LB agar (HiMedia). A bacterial count was performed after 18 h of incubation at 37°C.12 The experiment was repeated 3 times.
Adsorption of moxifloxacin to IDC tubes
According to the method of Reid et al., 1-cm sections of IDC tubing were placed in 3 test tubes containing moxifloxacin at concentrations of 100 µg/mL, 50 µg/mL and 25 µg/mL, and were incubated for 24 h at room temperature.13 After the incubation, the pieces were carefully washed with 2 mL of PBS (pH 7.1 ±0.1). The IDC pieces were then incubated in 2 mL of PBS (pH 7.0 ±0.1) for additional 60 min. Leakage of moxifloxacin into the solution from the IDC tubing was determined using the spectrophotometer analysis. The remaining moxifloxacin in the tube containing the IDC fragments (test) was estimated after 24 h of incubation at room temperature (spectrophotometric method). The decrease in concentrations of moxifloxacin indicates the amount of moxifloxacin bound to the IDC pieces. These amounts were compared with the level of moxifloxacin in control tubes (3 test tubes containing 100 µg/mL, 50 µg/mL and 25 µg/mL of moxifloxacin without IDC pieces). The larger the decrease in the concentration of the antibiotic, the better the adsorption of the antibiotic onto IDC pieces. The solutions were filtered through 0.2-µm pore-size Acrodisc filter units in Wheaton liquid chromatography vials. The quantity of moxifloxacin was calculated using maximum A290 values that were checked with UV Visible Scanning Spectrophotometer (Thermo Fisher Scientific, Waltham, USA). All experiments were repeated in triplicate.13
Adhesion of B. cepacia to tubes
coated with moxifloxacin
A similar procedure aimed at measuring the adhesion of B. cepacia to uncoated IDC tubes as mentioned above was followed. It allowed us to study the ability of B. cepacia to adhere to coated tubes in terms of the number (cfu/cm2) of B. cepacia that adhered to coated IDC tubes at different time intervals (1 h, 4 h and 24 h).
Statistical analyses
Calculations were performed to obtain a mean value and standard deviation (SD). The differences were analyzed by means of the Student’s t-test using Origin v. 8.0 software (OriginLab, Northamtpon, USA). A value of p < 0.05 was considered statistically significant.
Results
Adhesion of B. cepacia to IDC tubes
In this study, the kinetics of B. cepacia adhesion to pieces of uncoated IDC tubes at various time intervals (15 min, 1 h, 2 h, 4 h, 24 h, and 48 h) was investigated. The kinetics of B. cepacia adhesion to pieces of IDC tubes was confirmed by counting the viable bacterial count in cfu/cm2 (biofilm formation). The study showed the ability of the isolates to adhere to IDC tubes with high efficiency. A significant bacterial adhesion started 15 min after the incubation, with maximum adhesion observed at 48 h (Figure 1). The result of the present study showed a high and rapid ability of B. cepacia to adhere and form biofilm on IDCs.
Attachment of moxifloxacin
to pieces of IDC tubes
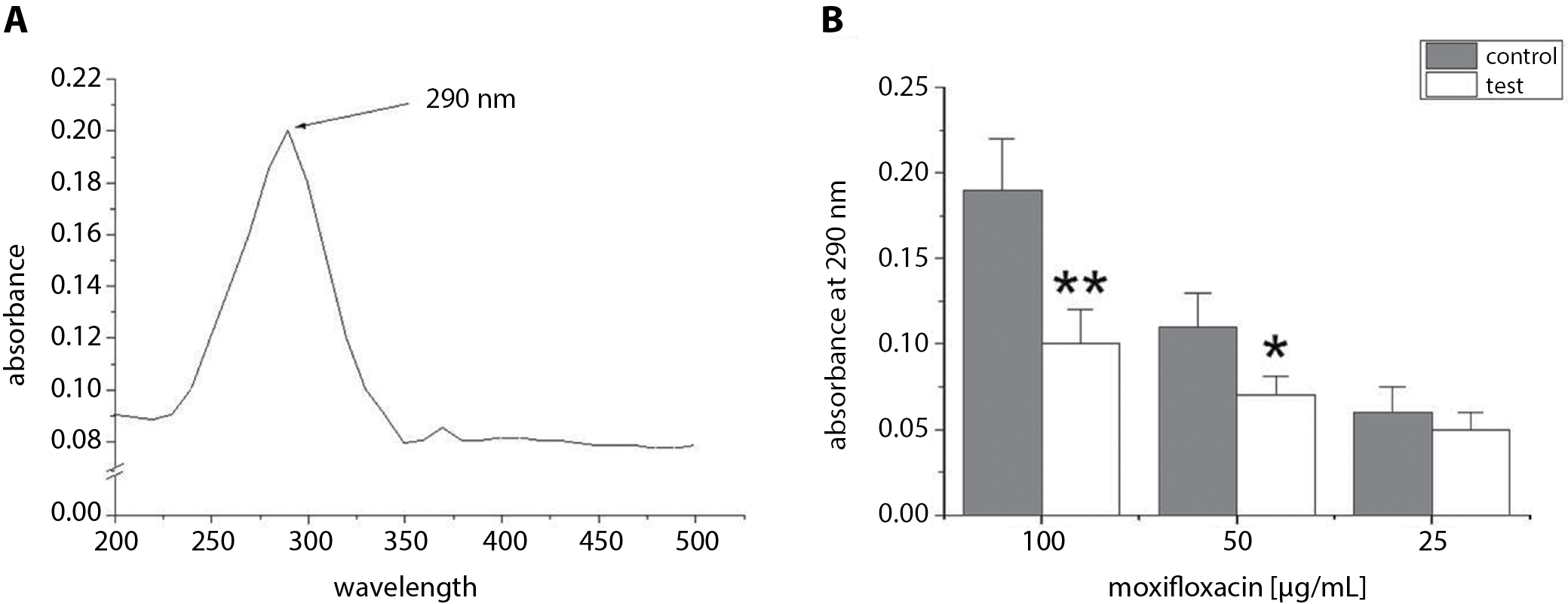
The optimum wavelength used in this experiment was estimated after studying different wavelengths to determine which one of them achieved the highest absorbance of moxifloxacin (100 µg/mL). Figure 2A shows that the maximum absorbance of moxifloxacin (100 µg/mL) was at 290 nm. This wavelength was used in further experiments to check the efficiency of adsorption of moxifloxacin to IDC tubes. The absorbance (at 290 nm) was measured in test tube solutions containing 100 µg/mL, 50 µg/mL or 25 µg/mL of moxifloxacin with IDC pieces. The test tube solutions were incubated for 24 h. The results were compared with control tubes (100 µg/mL, 50 µg/mL or 25 µg/mL of moxifloxacin without IDC pieces). Figure 2B showed that the absorbance of test tubes (50 µg/mL or 100 µg/mL of moxifloxacin and IDC pieces) at 290 nm was significantly lower than the absorbance of control tubes (50 µg/mL or 100 µg/mL of moxifloxacin without IDC pieces). No significant difference was observed between test tubes containing 25 µg/mL (p > 0.05) of moxifloxacin with IDC pieces and control tubes (25 µg/mL of moxifloxacin only). The present study showed that the best adsorption of moxifloxacin was observed after a 24-h exposure of the pieces of IDC to 100 µg/mL of moxifloxacin at room temperature. Thus, the deposition of moxifloxacin on catheters was dependent on moxifloxacin concentration. Therefore, the pieces of IDC exposed to 100 µg/mL of moxifloxacin were used to evaluate the effect of moxifloxacin-coated IDCs on B. cepacia adhesion and biofilm formation.
Role of moxifloxacin in reducing
bacterial biofilm formation
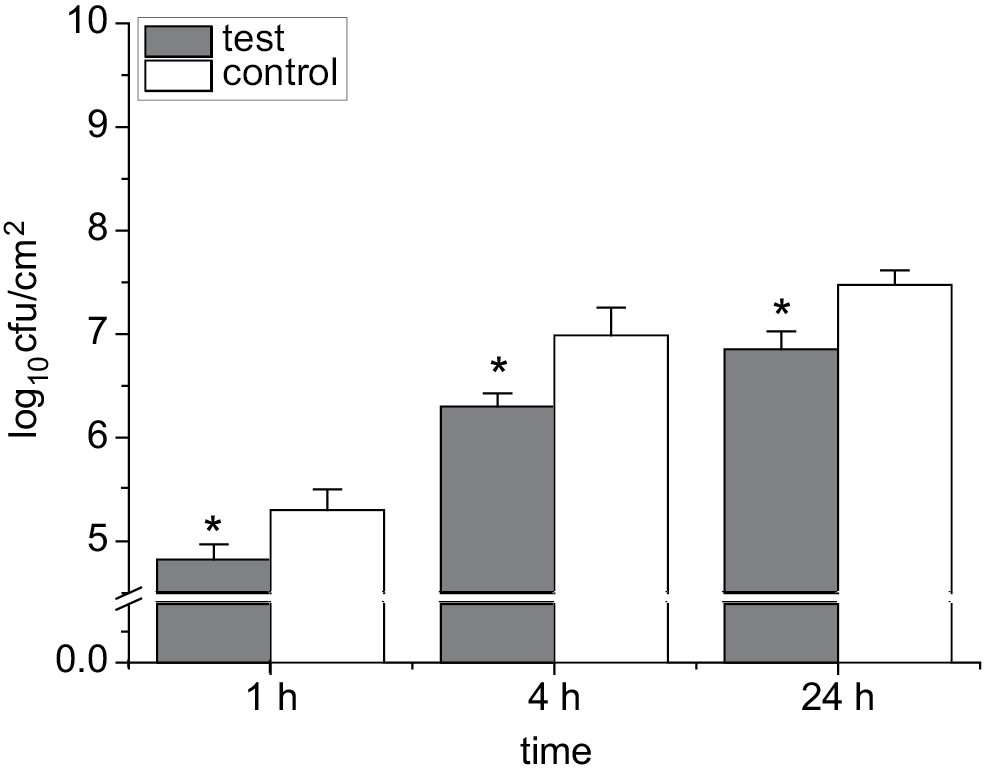
In the current study, the ability of B. cepacia to attach to coated IDC tubes with 100 µg/mL of moxifloxacin was evaluated. The effects of moxifloxacin-coated IDC tubes on the ability of B. cepacia to attach to these tubes were examined. This ability was evaluated at various time intervals (1 h, 4 h and 24 h). The results were compared with controls (uncoated pieces of IDC). Figure 3 shows a significant difference between the number of adhered bacteria (cfu/cm2) on coated and uncoated IDC tubes at all time points (1 h, 4 h and 24 h) after the incubation with bacterial suspensions (107 cfu/mL). The current study suggests that coating IDCs with moxifloxacin significantly reduces B. cepacia attachment to IDCs.
Discussion
Burkholderia cepacia can colonize moist surfaces and cause opportunistic infections, especially in intensive care patients. Multiple studies have shown that these bacteria maintain their vitality by forming biofilms and attaching to any surface rather than remaining in the planktonic form.3 Biofilms that form on biomaterials, medical devices, host epithelial cells, and mucosal surfaces cause serious medical and industrial problems as they become sources of contamination.13, 14 Studies have demonstrated that B. cepacia has the ability to adhere to a variety of plastic catheter surfaces by forming biofilms. This represents a big challenge in treating infections with these bacteria, especially in hospitalized patients who require IDC tubes as part of their medical care.8, 15 This study was aimed at finding a solution to this problem.
The current study showed a high and rapid ability of these bacteria to adhere to catheters. Its adherence was significant within only 15 min after the catheter was treated with a standard inoculum of B. cepacia. Our findings are consistent with the results of previous studies, showing that these bacteria have the ability to adhere and form biofilms on biotic and abiotic surfaces.12, 16 The ability of B. cepacia to adhere and form a biofilm is due to several factors including quorum sensing, as well as the effect of structural appendages such as flagella.12, 17
In the current study, a good method of adhesion elimination and biofilm formation of B. cepacia in IDC tubes was found. The method was dependent on coating IDC tubes with moxifloxacin. It was found that the adhesion of bacteria (B. cepacia) and biofilm formation were significantly reduced in the coated IDC tubes compared to uncoated IDC tubes. Our study is a pioneer research that successfully used coated IDC tubes with moxifloxacin to eradicate B. cepacia adhesion and biofilm formation. This project opens the door to new research aimed at manufacturing IDC tubes that do not allow bacteria to adhere and form a biofilm. Such feature of medical equipment could prevent the causes of dangerous infections in hospitalized patients.
Conclusions
Our study demonstrated the adhesion ability of B. cepacia to IDC tubes. Moreover, by coating IDCs with moxifloxacin, the ability of B. cepacia to adhere to IDC tubes and form biofilms was significantly reduced. We hope that these findings may help in reducing the chance of serious infections with B. cepacia, especially in patients suffering from kidney or urinary tract conditions.