Abstract
Background. Dental sealants are used to caulk fissures and pits in order to prevent caries development both in deciduous and permanent dentition. Loss of sealant integrity leads to the formation of marginal gaps, consequently increasing the risk of caries.
Objectives. This study aimed to compare the physicochemical and clinically relevant properties of 3 commercially available resin-based pit and fissure sealants: Arkona Fissure Sealant (AFS; Arkona, Nasutów, Poland), Flow-Color (FC; Arkona, Nasutów, Poland) and Flow-It ALC (FIA; Pentron, Orange, USA).
Materials and methods. After polymerization in dedicated molds, the materials were characterized using attenuated total reflectance Fourier-transform infrared spectroscopy (ATR-FTIR), surface free energy (SFE) measurements and micromechanical testing to evaluate structural and mechanical properties. Scanning electron microscopy (SEM) coupled with energy-dispersive spectroscopy (EDS) was employed to visualize sample morphology and determine elemental composition. An in vitro fluoride release study was conducted in artificial saliva at varying pH values (4.5, 5.5, 7.0, 7.5), with deionized water as a reference. Measurements were recorded at 1, 3, 24, 48, 72, and 96 h, and then weekly for up to 7 weeks.
Results. AFS exhibited the highest values of SFE (38.4 mJ/m2), Vickers hardness (51.93 HV) and indentation modulus (11.93 kN/mm2). All sealants demonstrated cumulative fluoride release over the incubation period, with the highest release observed for AFS in artificial saliva at pH = 7.5 (0.772 ppm). FTIR spectra of all materials confirmed the presence of polymer backbones as declared by the manufacturers.
Conclusions. Presented findings provide insight into material-dependent properties influencing adhesion, mechanical performance and ion release of resin-based dental sealants. Among the tested materials, AFS exhibited the most favorable overall profile, combining high filler content, optimized particle architecture, superior mechanical strength, elevated surface energy, and sustained fluoride release, which together support robust adhesion, resistance to occlusal forces and effective caries prevention.
Abstract (in Polish)
Wprowadzenie. Laki stomatologiczne są przeznaczone do uszczelniania bruzd i szczelin zębów w celu zapobiegania rozwojowi próchnicy zarówno w uzębieniu mlecznym, jak i stałym. Zmiany strukturalne zaaplikowanego laku prowadzą do utraty szczelności brzeżnej, co w konsekwencji zwiększa ryzyko rozwoju próchnicy.
Cel pracy. Celem badania była charakterystyka oraz porównanie właściwości fizykochemicznych, mechanicznych oraz adhezyjnych trzech komercyjnie dostępnych laków szczelinowych z matrycą polimerową: Arkona Fissure Sealant (AFS; Arkona, Nasutów, Polska), Flow-Color (FC; Arkona, Nasutów, Polska) oraz Flow-It ALC (FIA; Pentron, Orange, CA, USA).
Materiał i metody. Po fotopolimeryzacji materiałów w dedykowanych formach materiały poddano analizie za pomocą spektroskopii w podczerwieni (ATR-FTIR), pomiarom energii swobodnej powierzchni (SFE) oraz ewaluacji właściwości strukturalnych i mikromechanicznych. Morfologia próbek i ich morfologię oraz skład pierwiastkowy określono za pomocą elektronowej mikroskopii skaningowej (SEM) sprzężonej z dyspersyjną spektroskopią rentgenowską (EDS). Wyniki badania in vitro uwalniania fluoru przeprowadzono w sztucznej ślinie o różnych wartościach pH (4.5; 5.5; 7.0; 7.5) oraz w wodzie dejonizowanej jako próbie kontrolnej, z pomiarami wykonywanymi po 1, 3, 24, 48, 72, 96 godzinach oraz co tydzień do 7 tygodnia.
Wyniki. Wśród badanych materiałów AFS wykazał najwyższą wartość energii swobodnej powierzchni (38.4 mJ/m²), twardości Vickersa (51.93 HV) oraz modułu indentacji (11.93 kN/mm²). Wszystkie laki wykazały kumulatywne uwalnianie fluoru w trakcie inkubacji, przy czym najwyższe stężenie fluoru odnotowano dla AFS w sztucznej ślinie o pH 7.5 (0.772 ppm). Widma FTIR wszystkich materiałów potwierdziły obecność łańcuchów polimerowych zgodnie z deklaracjami producentów.
Wnioski. Przedstawione wyniki dostarczają wiedzy na temat właściwości wpływających na adhezję, właściwości mechanicznych oraz uwalniania jonów fluorkowych w lakach szczelinowych opartych na matrycach polimerowych. Spośród badanych materiałów AFS wykazał najbardziej korzystny profil, łącząc wysoką zawartość wypełniacza, morfologię powierzchni, wysoką wytrzymałość mechaniczną, podwyższoną energię powierzchniową oraz wysokie kumulatywne uwalnianie fluoru, co wspiera trwałą adhezję, odporność na siły okluzyjne oraz potencjalnie najskuteczniejszą profilaktykę próchnicy.
Key words: physico-chemical properties, caries prevention, fluoride release, dental fissure sealant, adhesion to dental tissues
Słowa kluczowe: właściwości fizykochemiczne, uwalnianie fluoru, stomatologiczny lak szczelinowy, prewencja próchnicy, adhezja do szkliwa
Background
Dental caries is a widespread, chronic, non-communicable microbial disease that can affect individuals at any age, resulting from a complex, multifactorial process that leads to the demineralization of dental tissues.1, 2 In the span of 20 years, it has affected approx. 2.4 billion individuals worldwide.3 Therefore, effective preventive strategies are essential, with fluoride-based interventions being the cornerstone of modern caries management. Fluoride exerts its anticaries effects through multiple mechanisms, including the inhibition of demineralization, promotion of enamel remineralization and suppression of bacterial
metabolism.4
Fluoride is capable of affecting dental health in preeruptive, posteruptive, systemic and topical mechanisms.5 On a structural level, it modifies the enamel surface by converting hydroxyapatite crystals into more chemically stable fluorapatite, enhancing resistance to acid attack and maintaining a protective barrier against cariogenic challenges.6
A variety of fluoride-containing products are available for caries prevention, including toothpastes, mouth rinses, gels, and sealants. Clinical evidence indicates that both fluoride varnishes and fluoride-containing fissure sealants are effective caries-preventive measures.7 However, the long-term clinical performance of fissure sealants is critically dependent on their ability to form a durable bond with dental tissues. Adhesion to enamel and dentin is influenced by a complex interplay of material properties, including surface energy, wettability, microstructural homogeneity, and chemical composition.
Variations in these physico-chemical characteristics can affect a material’s ability to penetrate fissures, adapt to the tooth surface, and withstand mechanical and chemical challenges in the oral environment. Furthermore, the chemical constituents of the sealant, including monomer composition, filler type and fluoride content, may modulate both the initial bonding efficiency and the sustained release of protective ions, thereby influencing the material’s anticariogenic potential.
Dental sealants are employed to caulk occlusal fissures, pits and foramina caeca to prevent caries development both in deciduous and permanent dentition. Nevertheless, their use is contraindicated in patients with known allergies or hypersensitivities to any component of the sealant material.4 Sealing is a painless procedure which may be carried out by professional dentists or dental hygienists. Dental sealants are divided into glass ionomer and resin-based materials. Among resin-based materials, ultraviolet-activated, auto-polymerized and light-cured formulations may be outlined.8 Materials for sealing pits and fissures are distributed under the name of dental sealants or composite or glass-ionomer materials with sealing indications. A systematic review by Azarpazhooh et al.8 indicates that resin-based sealants should be preferred by clinicians due to their better retention.
The effectiveness of fissure sealants depends not only on the intrinsic properties of the material but also on the method of application.9 Critical physico-chemical features include the ability to penetrate fissures and adhere strongly to enamel, while mechanical properties such as microhardness and wear resistance further determine long-term performance.10, 11 Among these factors, one of the most decisive for clinical success is the maintenance of marginal integrity throughout the service life of the sealant. Loss of marginal integrity leads to gap formation, providing niches for bacterial colonization and consequently increasing the risk of secondary caries.12
Furthermore, the efficacy of sealing is also dependent on anatomical factors. The morphology of occlusal fissures, pits and foramina caeca is a key factor in the retention rate of dental sealants. The geometry of fissures influences both the penetration and retention of the material.13 Finally, thinner enamel layer and wider dentinal tubules facilitate rapid progression of carious lesions, directly contributing to the accelerated development of the disease.14
A major mechanism underlying the loss of sealant integrity is polymerization shrinkage. During the curing process, monomers are converted into a cross-linked polymer network, which is accompanied by a reduction in material volume.15 When the contraction forces exceed the adhesive strength to enamel, micro-separation may occur at the interface between the sealant and the tooth surface. The resulting microgaps create favorable conditions for bacterial penetration, thereby facilitating the initiation and progression of carious lesions in the compromised area.16
Despite the proven effectiveness of fissure sealants in caries prevention, certain limitations remain regarding their long-term application. The most prominent challenge is the loss of marginal integrity at the material–enamel interface, which contributes to gap formation and reduces the durability of the protective barrier. For this reason, ongoing research is focused on identifying material and application strategies that can minimize shrinkage effects, improve adhesion, and ensure a stable and effective seal over time.
Objectives
The objective of this study was to make a comparison between the physicochemical and clinical-relevant properties of 3 commercially available resin-based pit and fissure sealants. The following resin-based sealants were examined: Arkona Fissure Sealant (AFS; Arkona, Nasutów, Poland), Flow-Color (FC; Arkona), and Flow-It ALC (FIA; Pentron, Orange, USA).
Materials and methods
In the present study, 3 commercially available composite resin-based materials for pit and fissure sealing (AFS, FC and FIA) were selected and subjected to a comprehensive experimental evaluation. The assessment included physico-chemical characterization by attenuated total reflectance Fourier-transform infrared spectroscopy (ATR-FTIR) and water contact angle (WCA) measurements, as well as the determination of mechanical and physical properties relevant to clinical performance. In addition, the fluoride release potential of the tested materials was investigated in order to assess their anticariogenic capacity. A detailed overview of the materials, including their composition and manufacturer-reported characteristics, is provided in Table 1, while their macroscopic appearance is illustrated in Figure 1.
Specimen preparation and experimental design
Since fissure sealants are originally liquid polymeric materials, polymerized specimens were prepared for the experimental procedures. Two main groups of cylindrical samples were fabricated to assess the physico-chemical, mechanical and functional properties of the tested
materials.
The 1st group consisted of cylinders with a diameter of 12 mm and a height of 5 mm, dedicated to physico-chemical analyses. The 2nd group comprised smaller specimens (5 mm in diameter and 2 mm in height), prepared in dedicated polytetrafluoroethylene (PTFE) molds,20 specifically designed for the evaluation of fluoride ion release. All specimens were produced under identical conditions and light-cured strictly according to the manufacturers’ instructions: AFS and FIA for 10 s, and FC for 30 s, using an LED curing lamp Elipar II (3M ESPE, St. Paul, USA).
For physico-chemical characterization, due to the requirement of flat and parallel surfaces, each polymerized specimen was subsequently embedded in heat-curing acrylic resin (Duracryl® Plus). The embedded samples were sectioned using a precision metallographic cutter (STRUERS® Accutom-5) and carefully polished to obtain reproducible, smooth surfaces suitable for measurement. These prepared specimens were then subjected to a set of analyses, including microhardness testing, surface morphology evaluation using scanning electron microscopy (SEM) coupled with energy-dispersive X-ray spectroscopy (EDS), Fourier-transform infrared spectroscopy (FTIR), and determination of contact angle and surface free energy.
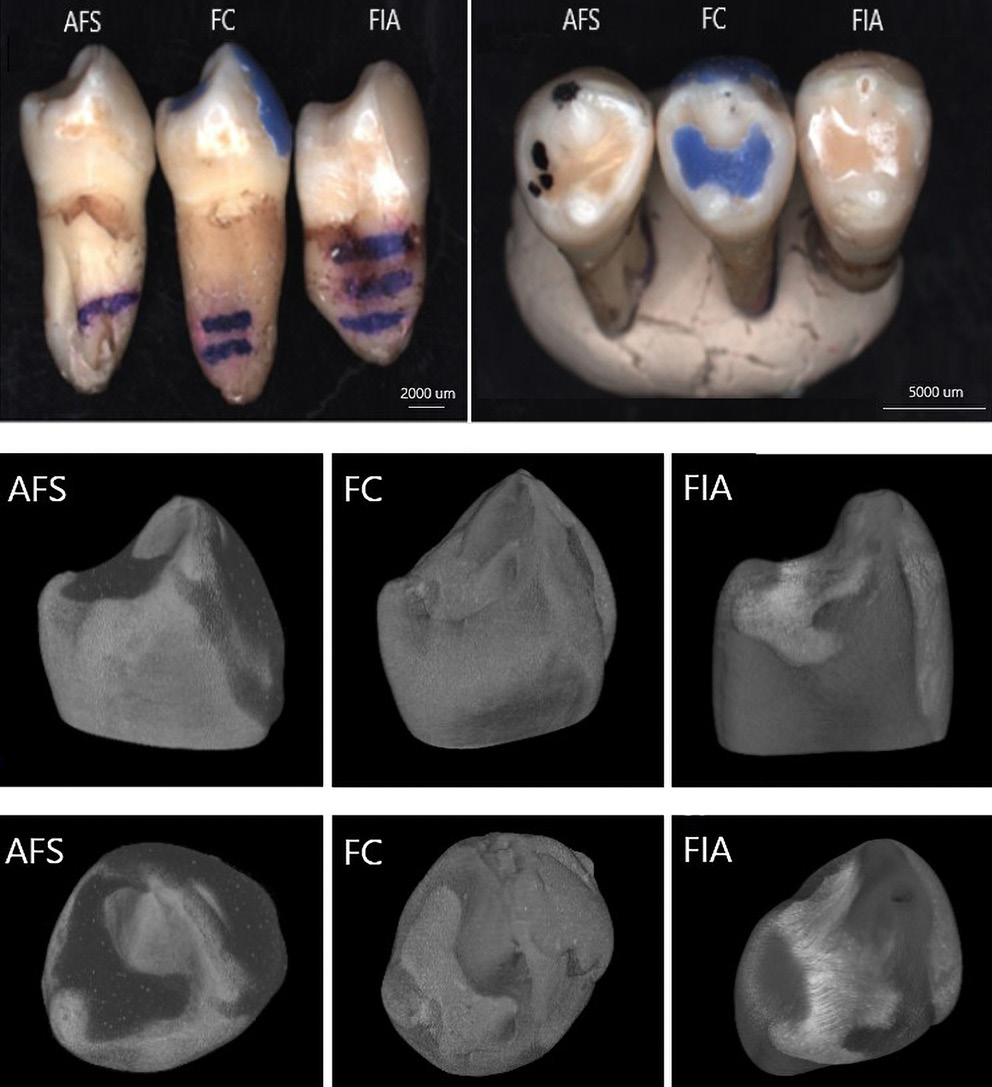
In order to investigate the adhesion of fissure sealants to dental tissues, extracted premolars were included in the study. The teeth were prepared in accordance with clinical recommendations, and sealants were applied to the pits and fissures of the occlusal surfaces of the premolar’s crowns (Figure 2). Following polymerization, the sealed teeth were subjected to advanced imaging and characterization techniques, including micro-computed tomography (micro-CT) and SEM, to visualize and evaluate the interfacial adaptation and integrity of the material–tooth
interface.
ATR-FTIR
The ATR-FTIR analysis was performed on cured sealant samples. Spectra were acquired using a Nicolet iZ10 infrared spectrometer (Thermo Fisher Scientific, Waltham, USA) equipped with an ATR accessory. The measurements spanned a spectral range of 4000–550 cm−1, with a resolution of 4 cm−1, and each spectrum was averaged over 32 scans.
Scanning electron microscopy
The surface morphology of the tested fissure sealants was examined using a SEM (Phenom ProX; Thermo Fisher Scientific Observations were carried out under high-vacuum conditions at various magnifications, which enabled a detailed assessment of both surface topography and the homogeneity of the investigated materials. The evaluation focused on the presence of morphological features such as pores, cracks, material discontinuities, and the quality of adhesion between the sealant and the underlying substrate. To complement the structural observations, an elemental composition analysis was performed using EDS. This approach allowed for the identification and qualitative assessment of the main chemical elements present on the surface of the fissure sealants, thereby providing additional insight into the material characteristics relevant to their clinical performance.
Surface wettability analysis
To quantitatively characterize the wettability of the investigated sealants, contact angle measurements and subsequent determination of surface free energy (SFE) were performed, providing insight into the hydrophilic or hydrophobic character of the tested materials. The method relied on the analysis of the shape of a liquid droplet deposited on the sample surface under controlled conditions. Measurements were conducted using an optical contact angle goniometer (Surftense Universal; OEG GmbH, Frankfurt am Main, Germany). The surface free energy was calculated using the Owens–Wendt approach,21 which is based on contact angle values obtained for at least 2 probe liquids of known surface tension parameters. The surface energy components of the different liquids used in this study are summarized in Table 2.
This method allows the decomposition of SFE into its dispersive and polar components, thereby enabling a comprehensive assessment of the surface characteristics and their potential influence on adhesive interactions. For each specimen, 5 independent measurements were performed using 2 probe liquids: distilled water (polar liquid) and diiodomethane (non-polar liquid, purity 99%). A single droplet of 0.80 ±0.01 μL of the respective liquid was carefully dispensed onto the polished specimen surface for each measurement, and the contact angle was recorded within 1 s after droplet deposition to minimize the effects of evaporation or spreading.
Microhardness testing
Hardness is one of the fundamental parameters of dental materials, reflecting their resistance to localized deformation and surface wear. Microhardness and reduced elastic modulus were evaluated using instrumented indentation in accordance with ISO 14577, employing a Berkovich indenter (65.3° apex angle, <150 nm tip radius) mounted on a MicroCombi Tester (CSM Instruments SA, Peseux, Switzerland). The measurements were carried out under a load of 400 mN, and data acquisition was performed following the Oliver–Pharr method. This approach enabled not only the determination of Vickers hardness values, but also provided detailed insight into the mechanical response of the material during deformation. In this procedure, after reaching the maximum load, the indenter was held in contact with the specimen for a defined dwell time, producing a characteristic load–displacement curve.23 From this curve, a comprehensive set of mechanical parameters was derived, including Vickers hardness (HV), instrumented hardness (HIT), reduced modulus of elasticity (Er), instrumented modulus of elasticity (EIT), work ratio (ηIT) and indentation depth (hr) and at peak load. A minimum of 5 indentations (n = 5) were performed per area. All measurements were conducted at room temperature (~22°C) and relative humidity of 40–50%.
Fluoride release
Fluoride ion release was evaluated using an Orion 9609 ion-selective electrode (Thermo Fisher Scientific) connected to a CPI-551 pH/ion meter (Elmetron, Zabrze, Poland). Prior to each measurement, the system was calibrated with standard fluoride solutions to ensure accuracy and reproducibility. The electrode setup was subsequently employed to monitor fluoride ion leaching from the sealant matrices under different experimental conditions. Fluoride release was assessed at the following time intervals: 3, 24, 48, 72, 96, and 168 h, with 4 independent replicates per material. The release was determined in both deionized water and artificial saliva solutions at different pH values (4.5, 5.5, 7.0, and 7.5). The artificial saliva formulation, based on previously reported protocols,24, 25 did not contain calcium ions in order to avoid precipitation phenomena. It was prepared in deionized water and consisted of the following components: 0.4 g/L sodium chloride (NaCl), 0.4 g/L potassium chloride (KCl), 0.005 g/L sodium bisulfate (Na₂S·9H₂O), 0.78 g/L sodium dihydrogen phosphate (NaH₂PO₄·2H₂O), and 1.0 g/L urea. All reagents were supplied by Chempur (Piekary Śląskie, Poland). The initial burst release (IBR) was defined as the fraction of the cumulative fluoride released within the first 24 h relative to the total cumulative fluoride release index (CFRI) after 7 weeks. The half-release time (t1/2) was determined as the time range at which 50% of the total fluoride release had occurred.
Adhesion to dental tissues
To evaluate the adhesion of fissure sealants to dental tissues, extracted premolars were employed. The occlusal fissures of the teeth were sealed following standard clinical protocols using the tested materials. The prepared specimens were subsequently analyzed using micro-CT (1172 SkyScan; Bruker, Kontich, Belgium) to assess interfacial adaptation. Scanning parameters were set at 95 kV and 100 μA, with a rotation step of 0.21° over a full 360° rotation, using a 0.5 mm Al filter and an exposure time of 2,000 ms per projection.
Additionally, SEM was performed to evaluate marginal sealing and identify potential micro-gaps at the enamel–sealant interface. This dual approach allowed for comprehensive visualization of both the internal interfacial integrity and the surface morphology at high resolution.
Statistical analyses
All obtained data were subjected to statistical analysis using OriginPro 2025 (OriginLab Corporation, Northampton, USA) and Microsoft Excel 2013 (Microsoft Corporation, Redmond, USA). For each parameter, including mechanical properties, surface characteristics and fluoride release, mean values and standard deviations (SDs) were calculated to summarize the central tendency and variability of the data. The distribution of the datasets was evaluated, and because some datasets deviated from normality (p < 0.05), nonparametric statistical testing was performed. Statistical significance was assessed at a threshold of p < 0.05 using the Mann–Whitney U post hoc test. For cumulative fluoride release data, error values were calculated as the square root of the sum of the squares of the individual SDs, assuming that errors at each time point were independent. This approach allowed for a reliable assessment of differences between materials while accounting for variability across measurements.
Results
Morphological and physico-chemical characterization
Surface investigations of the sealants were conducted using SEM equipped with EDS, as well as ATR-FTIR.
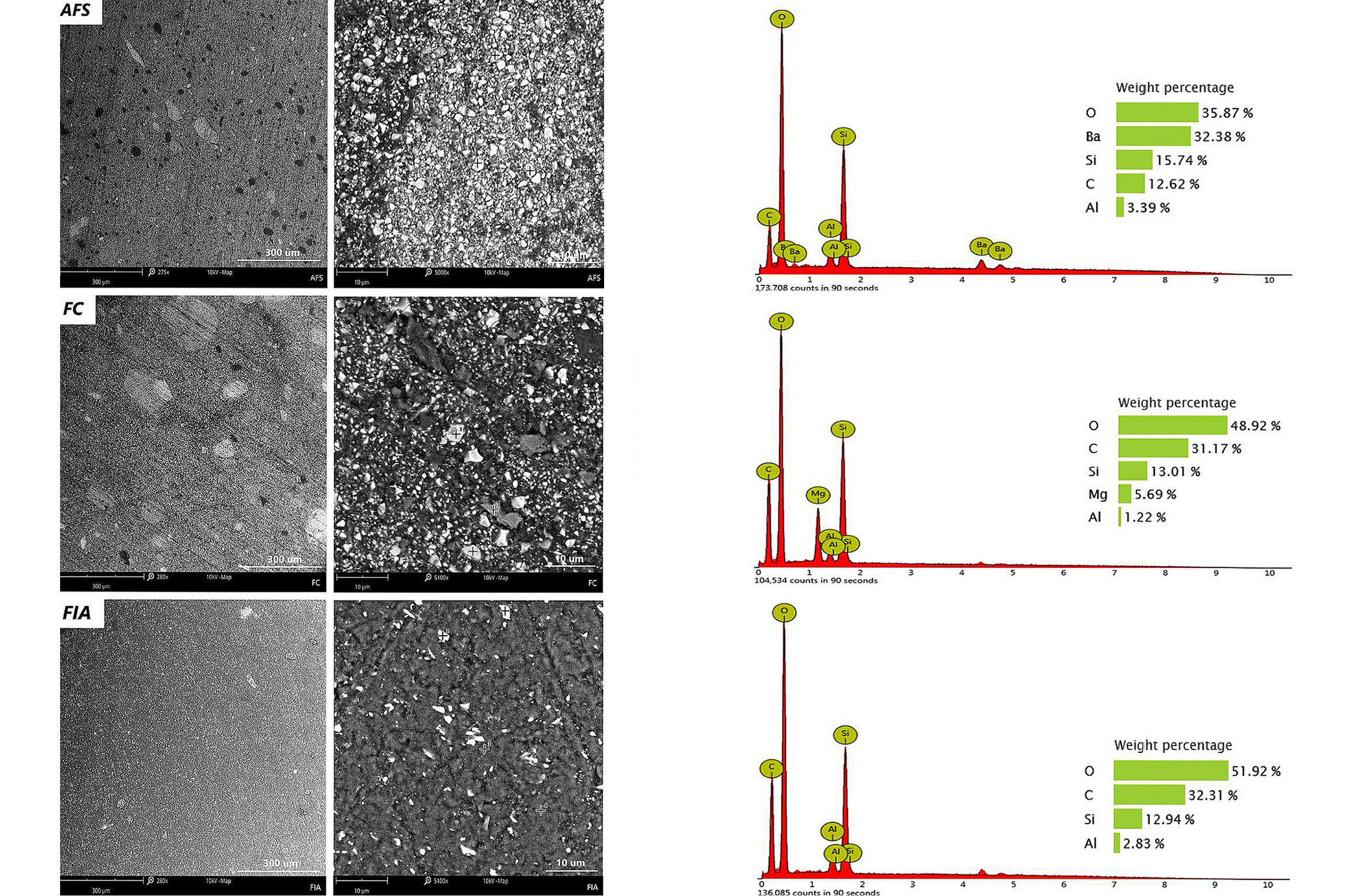
Scanning electron microscopy observations performed at magnifications of ×280 and ×5,000 revealed that the analyzed surfaces were smooth and continuous, without the presence of microcracks within the polymerized material. At higher magnification (×5,000), particularly in AFS and FC sealants, the structure appeared fine-crystalline, containing particles of approx. 0.5–1 μm in size. Spectroscopic analysis identified these particles as silicate phases. The presence of silicon and aluminum in the sealants is most likely derived from silica (SiO₂), which is a commonly applied inorganic filler in dental composite.26 In addition, AFS and FC materials demonstrated minor amounts of titanium and barium, suggesting the incorporation of additives aimed at enhancing radiopacity of the dental materials. In contrast, FIA sealant exhibited the highest carbon content (42.9%) and simultaneously the lowest oxygen content (48.8%), with significantly lower silicon content (6.87%) compared to the other tested materials (Figure 3). The more diversified elemental composition observed in AFS and FC relative to FIA may account for differences in their physico-chemical performance.
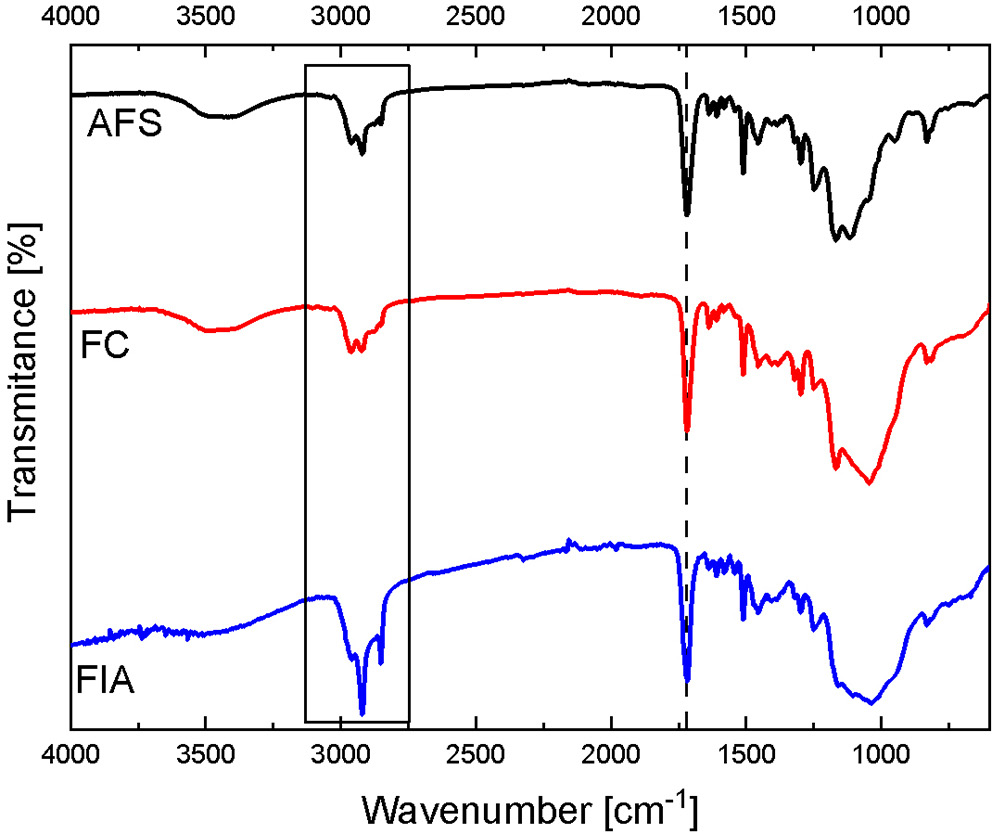
All evaluated fissure sealants contained various methacrylate derivatives forming their resin matrix (Table 1). The primary differences among the materials relate to their filler type and additional components, which are responsible for variations in clinical performance. FTIR spectra of all specimens (Figure 4) exhibited a characteristic band at ~1,700 cm–1, corresponding to the C=O stretching vibration of the methacrylate carbonyl group. Minor shifts in this band reflect the use of different methacrylate derivatives. The spectral region between 2,750 and 3,000 cm–1 displayed overlapping C–H stretching vibrations from alkane and aldehyde groups, confirming the aliphatic nature of the polymer matrix.
The fingerprint region below 1,500 cm–1 is distinct for each sealant and depends on the specific composition and relative amounts of fillers and additives. Due to the complexity of the formulations, a detailed quantitative analysis of all peaks was not performed.
Surface wettability and surface free energy
Contact angle measurements provided valuable information regarding the hydrophilicity of the tested fissure sealants (Table 3). According to standard interpretation, a contact angle below 90° indicates a hydrophilic surface with affinity for water, whereas a contact angle above 90° suggests a hydrophobic surface that tends to repel water. Based on the measured contact angles, the surface free energy components were calculated using the Owens–Wendt method,21 including the dispersive component (γSd), polar component (γSp) and total surface free energy (γS).
AFS sealant exhibited the lowest contact angles, indicating relatively higher wettability. In contrast, FC and FIA showed higher contact angles, particularly with distilled water, suggesting a larger contribution of the dispersive component and consequently lower hydrophilicity. Analysis of the surface energy components confirmed these observations. The total surface energy of AFS was the highest at 55.1 mJ/m², while FC showed the lowest value of 50.7 mJ/m². The higher wettability and adhesive potential of AFS may be important for interactions with dental tissues. Differences between FC and FIA were less pronounced, but may reflect variations in the surface structure of the tested materials. The FIA sealant exhibited the highest polar ratio (Ps) values, which are associated with its fine-crystalline and homogeneous structure. This finding also indicates that FIA possesses the greatest ability to attract polar molecules, such as water.
Mechanical properties
The mechanical performance of 3 commercially available fissure sealants (AFS, FC and FIA) was evaluated using instrumented indentation tests (Table 4). Parameters assessed included indentation depth (hr), Martens hardness (HM and HIT), indentation modulus (EIT), and work ratio (ηIT). These tests allowed quantification of each sealant’s resistance to mechanical deformation and its elastic response under load.
The mechanical and indentation tests showed clear differences among the 3 fissure sealants. AFS exhibited the lowest indentation depth 5.14 µm and the highest hardness (HM: 396.74 N/mm²; HIT = 545.55 N/mm²), indentation modulus (EIT = 11.93 kN/mm²) and work ratio (ηIT = 37.44%), indicating superior mechanical resistance. FC showed intermediate values across all parameters (h = 7.78 µm; HM = 183.05 N/mm²; HIT = 246.50 N/mm²; EIT = 5.97 kN/mm²; ηIT = 30.69%), while FIA presented the highest indentation depth (hr = 10.00 µm) and the lowest hardness and modulus values (HM = 111.32 N/mm²; HIT = 149.70 N/mm²; EIT = 3.83 kN/mm²; ηIT = 29.46%), indicating the weakest mechanical performance. In our study, mechanical characterization was carried out using both traditional indentation (yielding HV and HIT values) and instrumented indentation testing, which additionally provided Martens hardness (HM). Unlike conventional Vickers hardness, HM is derived from the continuous recording of applied force and indentation depth, making it less sensitive to the selected test load and thereby more reproducible. For dental materials with a composite structure of resin matrix and inorganic fillers, HM offers a more comprehensive assessment of mechanical performance, as it accounts for both elastic and plastic responses
of the material.
Statistical analysis (Mann–Whitney test) confirmed that most differences between sealant types were significant (p < 0.05), with superscript letters in Table 4 denoting pairwise significant differences. Overall, these results suggest that AFS has the highest mechanical resistance, FC is intermediate, and FIA shows the lowest mechanical performance among the tested fissure sealants.
Fluoride release
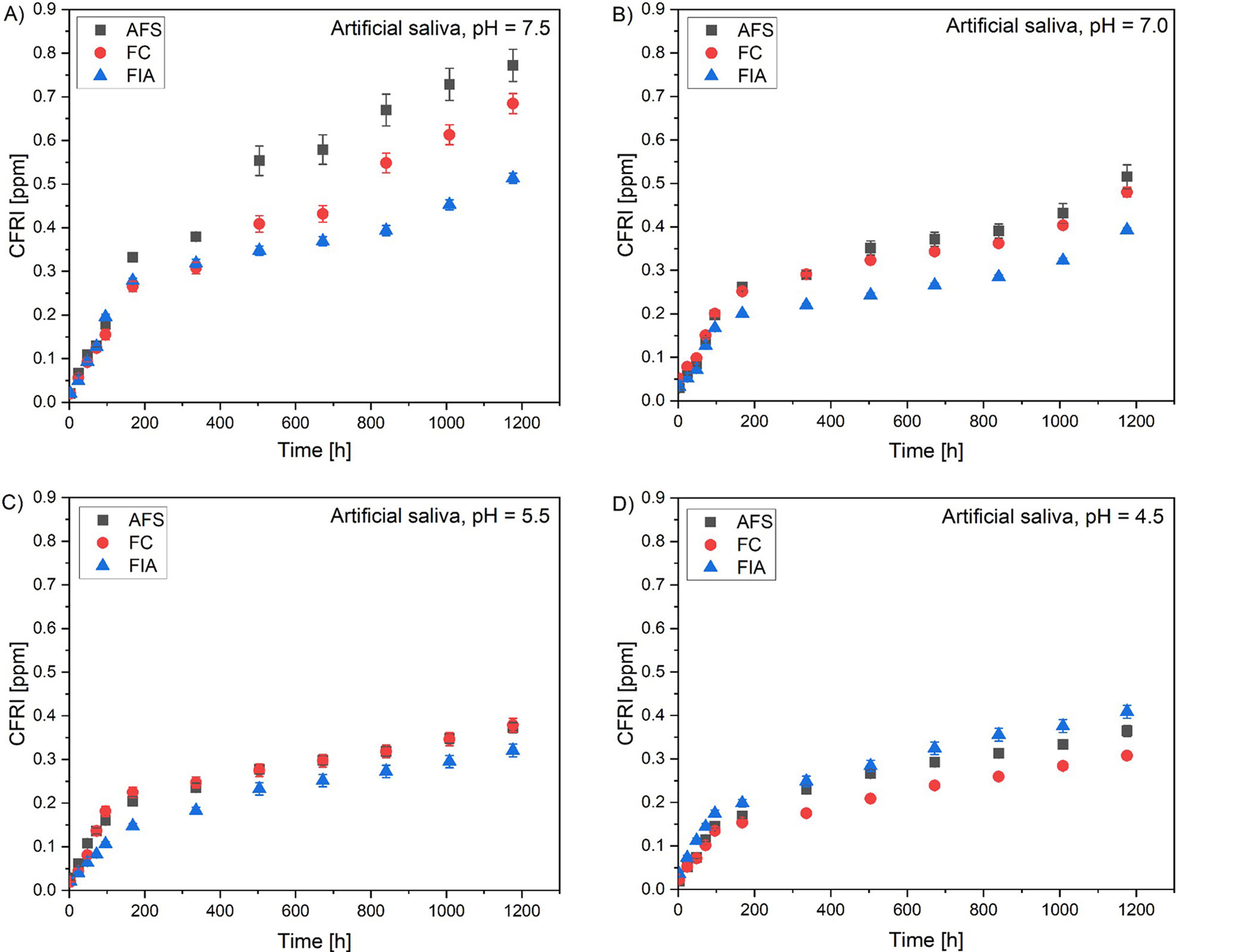
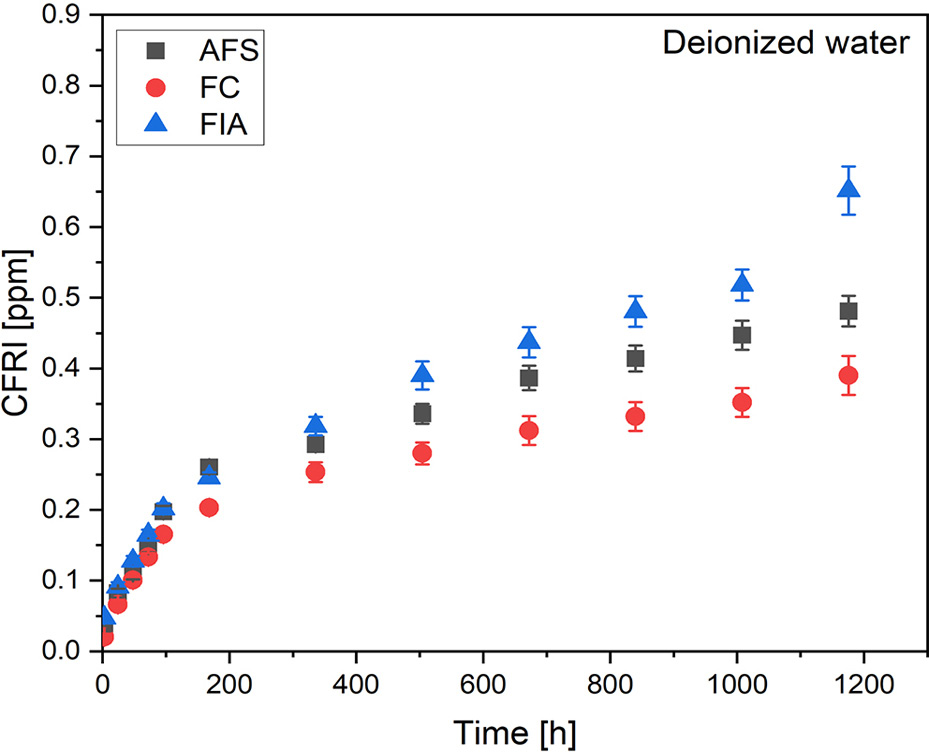
The release of fluoride was evaluated in artificial saliva (pH = 4.5, 5.5, 7.0, 7.5) and deionized water for all the sealants that were analyzed. The evaluation was conducted over a period of 3, 24, 48, 72, 96 h, and then once a week for a period of 7 weeks. The values of the CFRI are presented in Figure 5 for the artificial saliva solution and in Figure 6 for the deionized water.
The cumulative fluoride release index (CFRI) value demonstrated an upward trend over time for every solution and every evaluated sealant. Results indicate highest fluoride release in the 1st week of incubation. After 7 weeks of incubation, AFS possessed the highest CFRI in artificial saliva with pH = 7.5 (0.772 ppm) and pH = 7.0 (0.516 ppm). The pH of 5.5 highest CFRI value was comparable for AFS (0.373 ppm) and FC (0.378 ppm). As per artificial saliva with pH = 4.5 and deionized water, highest CFRI was assessed for FIA – 0.408 ppm and 0.651 ppm, respectively.
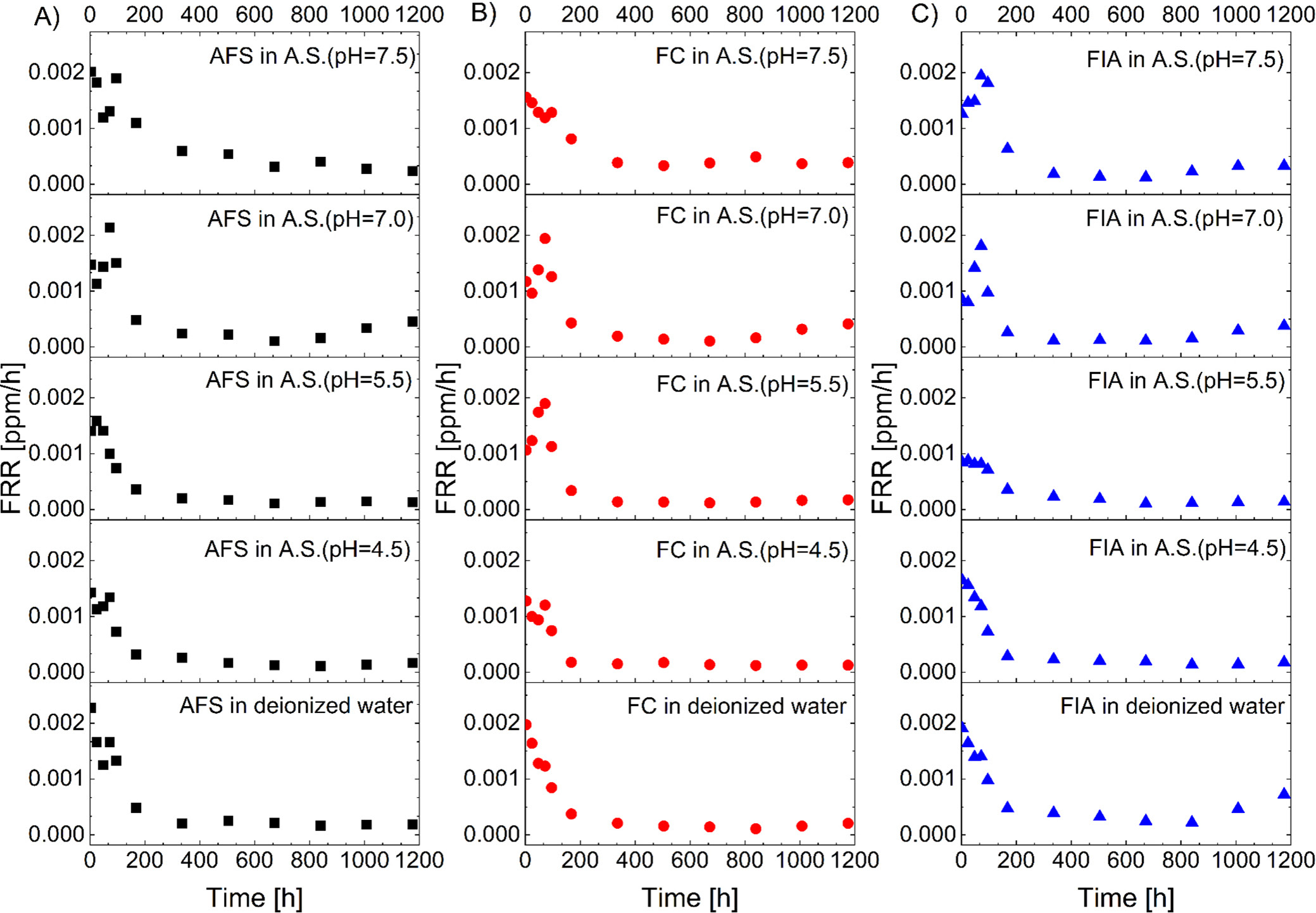
Fluoride release rate (FRR) profiles were derived from the 1st derivative of the CFRI with respect to time for all tested solutions and materials (Figure 7). For the purpose of comparative evaluation, the following parameters were extracted: IBR, maximum FRR, time at maximum FRR, t1/2, and cumulative release values at 7 weeks (Table 5).
The IBR after 24 h exhibited variation across evaluated solutions and sample types (AFS, FC, FIA), ranging from 8.2% to 17.9%. The maximum FRR observed ranged from 0.0010 to 0.0025 ppm/h, with the time to reach maximum release occurring primarily at either 3 h or 72 h. The t1/2 occurred at 96–168 h, 1–2 and 1–3 weeks’ time ranges, while the CFRI at 7 weeks ranged between 0.308 and 0.772 ppm.
Specifically, in artificial saliva with a pH of 7.5, the IBR values were lowest (8.2–9.5%), with maximum release mostly occurring after 3 h, and a half-life of 2–3 weeks for AFS and FC, and 96–168 h for FIA. The CFRI values after 7 weeks were the highest, reaching 0.772 ppm for AFS. In artificial saliva with pH = 7.0, the IBR increased to 12.3–16.3%, with maximal release at 72 h and a half-life of 96–168 h. However, the maximum CFRI decreased. At lower pH values (5.5 and 4.5), the IBR remained elevated (11.6–17.9%) with variable timing of maximum release and shorter half-lives, mostly between 1 and 2 weeks. In deionized water, the IBR was in the upper range (14.0–17.2%), with a rapid maximum FRR at 3 h, while the half-lives were similar to those at higher pH values. Overall, the results demonstrate that the IBR, release kinetics, and half-life are influenced by both the pH and the surrounding medium. The highest initial burst and highest FRR were observed in artificial saliva and deionized water at lower pH values, while cumulative release after 7 weeks tended to decline under these conditions.
Adhesion of fissure sealants to enamel surfaces
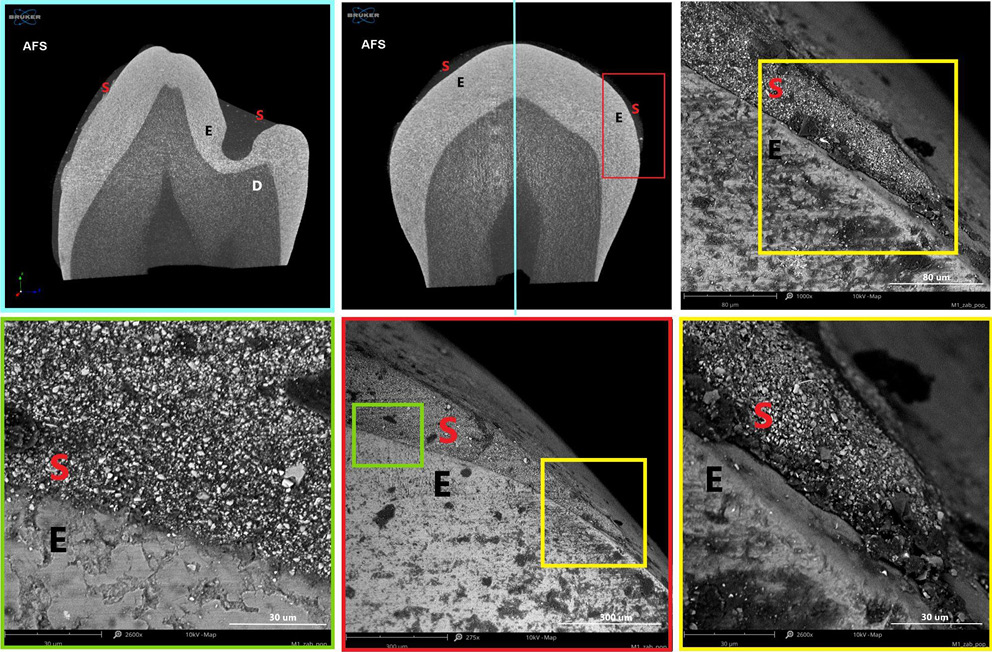
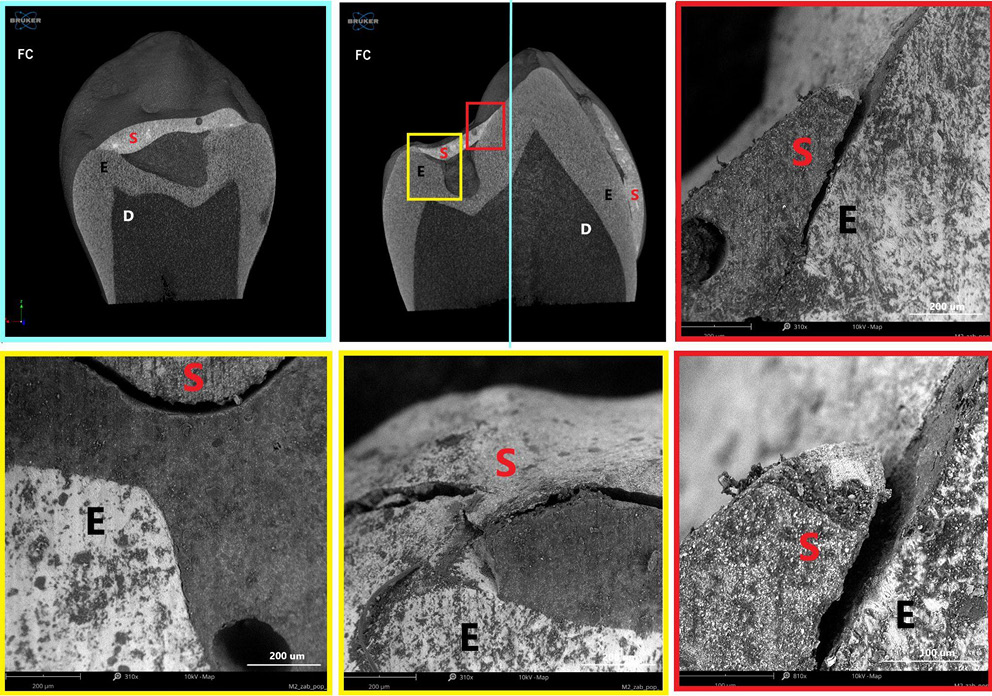
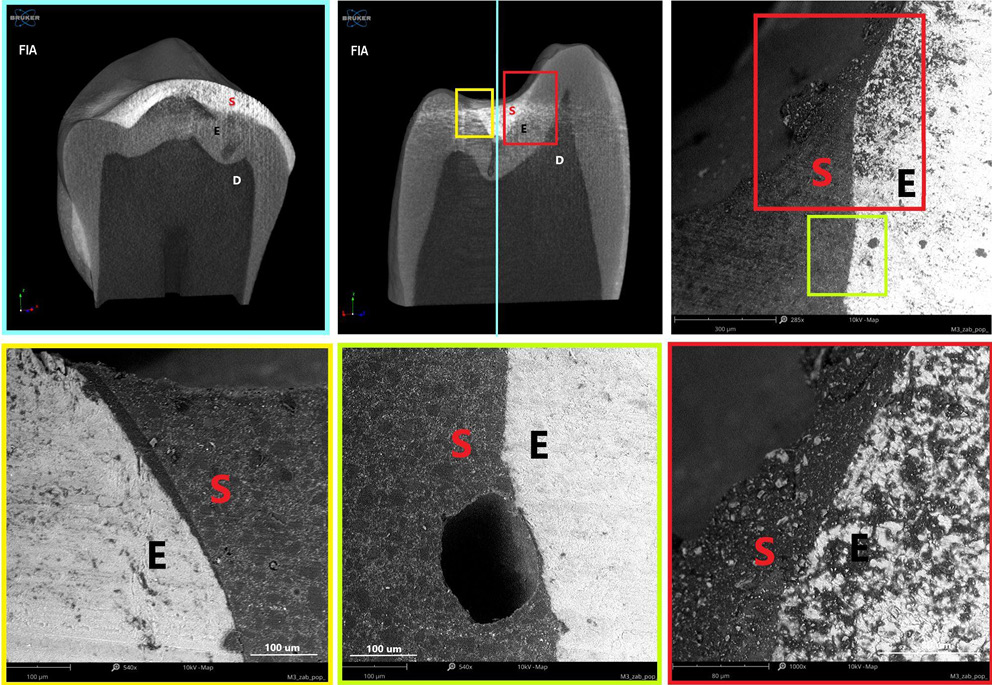
Polymerization shrinkage represents a critical limitation of dental restorative materials, as it can induce microcracks and interfacial gaps due to both intrinsic polymerization and thermal stresses. The material’s microstructure, in combination with its elastic properties, facilitates optimal adaptation and intimate contact with the enamel surface, promoting effective adhesion. Figure 8–Figure 10 present micro-computed tomography (micro-CT) reconstructions alongside SEM images of premolars filled with AFS, FC and FIA sealants. Sagittal and frontal cross-sections reveal the internal structure of the materials, while magnified views highlight the interface between the sealant and enamel. Examination of these sections clearly demonstrates the presence of cracks, pores and marginal gaps, which arise from polymerization-induced shrinkage and thermal stresses.
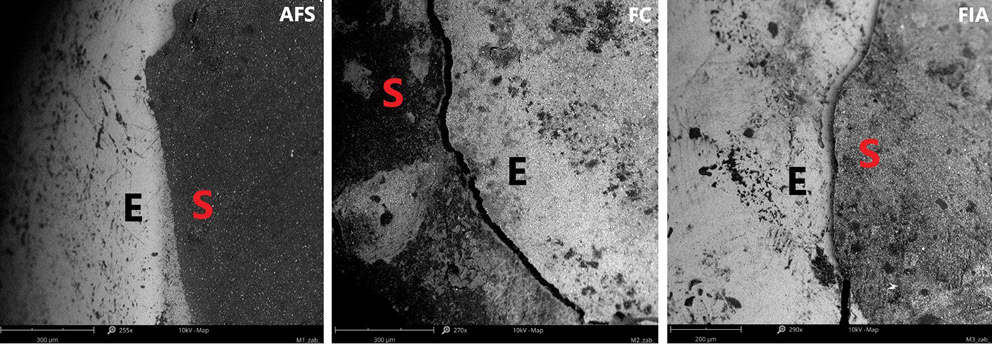
Figure 11 provides a top-down view of the sealant–enamel interface, showing an overlay-type connection characterized by structural discontinuities. These discontinuities are likely associated with local variations in wear properties. Given the higher stiffness and hardness of enamel relative to the sealant, the resin layer is expected to undergo initial wear during occlusal loading. From a mechanical perspective, the formation of such an interface, particularly during polymerization, can create localized stress concentrations, potentially promoting crack initiation associated with shrinkage.27 Effective adaptation to the enamel surface in pit and fissure sealants also relies on micromechanical interlocking with acid-etched enamel, which creates microscopic porosities on the tooth surface. The resin penetrates these micropores, forming a stable physical bond between the enamel and the sealant.26 This micromechanical connection is crucial for enhancing sealant retention and minimizing microleakage.
According to the manufacturer, the polymerization shrinkage of AFS ranges from 3% to 7%. In contrast, flowable materials,28 despite their higher viscosity and flowability, may exhibit polymerization shrinkage 1–2% greater than conventional Bis-GMA-based dental resins.29 This increased shrinkage contributes to wider interfacial gaps, which can act as microleakage pathways.
These microenvironments facilitate the accumulation of microorganisms, particularly in areas that are difficult to clean, potentially leading to subsurface carious lesions beneath the sealant. Such findings underscore the influence of the material’s elastic properties, filler content and microstructure on its ability to adapt to enamel surfaces and achieve effective adhesion. Consequently, the formation of interfacial gaps not only compromises marginal integrity but also increases the risk of secondary caries, underscoring the importance of optimizing material composition, filler architecture and application techniques to minimize polymerization shrinkage, thermal stress and related clinical complications.
Discussion
The present study provides a comprehensive evaluation of the physico-chemical, morphological, mechanical, and fluoride-release properties of 3 commercially available dental sealants (AFS, FC, FIA), highlighting how these characteristics directly influence clinical performance. The long-term effectiveness of pit and fissure sealants is strongly determined by their surface properties, mechanical behavior and fluoride release, which collectively govern adhesion, retention and caries-preventive potential.
The quality of marginal sealing is critically influenced by both intrinsic material properties and enamel preparation methods. Polymerization shrinkage, for instance, plays a major role in marginal adaptation. Kucukyilmaz et al.30 reported that sealants with higher filler content, such as Helioseal F, exhibited lower volumetric shrinkage (~3.3%), whereas materials with lower filler load, such as Teethmate F-1, reached ~7.4%. These findings emphasize the importance of filler fraction in minimizing interfacial gaps and preserving long-term adhesion. Surface conditioning is equally crucial; Amend et al.28 demonstrated that enamel etching with 37% phosphoric acid or using Clearfil SE Bond produced tighter marginal sealing and lower microleakage than self-etching primer systems, as confirmed by dye penetration and SEM imaging. Procedural variations also affect sealant retention. Chaitra et al.31 compared conventional preparation, enameloplasty, and fissurotomy, showing that enameloplasty significantly reduced microleakage and generated longer resin tags (~12 µm vs ~6 µm for other methods). Adhesive performance under suboptimal conditions is another determinant; Memarpour et al.32 observed that saliva contamination disrupted mechanical interlocking and increased gaps at the enamel–sealant interface, highlighting the importance of contamination-free application or corrective adhesive protocols. Together, these studies support our observation that AFS systems, by design, provide superior adhesion
and marginal sealing.
Excessive polymerization shrinkage and thermal stresses are well-known contributors to interfacial gap formation.33 Our study, employing high-resolution micro-CT combined with SEM, allowed detailed visualization of cracks and interfacial gaps induced by polymerization. These defects are not merely structural artifacts; they may serve as pathways for microleakage, facilitating microbial infiltration into the fissure system. Once microorganisms penetrate these gaps, they can proliferate, forming biofilms that are difficult to remove during routine oral hygiene. Such colonization compromises sealant integrity and increases the risk of secondary caries, underscoring the clinical importance of minimizing shrinkage and thermal stress through material selection and optimized application techniques. Correlating micro-CT and SEM data enabled precise mapping of the spatial distribution and dimensions of these defects, providing mechanistic insight into how polymerization-related stresses translate into functional vulnerabilities. Therefore, the internal structure and filler architecture of sealants appear to be key determinants in mitigating such effects.
Elemental analysis using EDS revealed that AFS and FC contained higher concentrations of silicon and aluminum, indicative of a greater inorganic filler content. Scanning electron microscopy imaging confirmed a granular, rough surface morphology with visible filler agglomerates for these materials, correlating with enhanced mechanical properties, potentially increasing susceptibility to biofilm formation.34, 35 FIA, in contrast, exhibited fewer inorganic components, lacked detectable aluminum and titanium, and presented a smoother, more homogeneous surface with loosely packed filler particles and void spaces. These morphological characteristics explain the lower mechanical resistance and higher indenter penetration observed for FIA.
The mechanical performance of resin composites is largely dictated by filler content, size and geometry.36 Early studies by Li et al.37 established that both filler fraction and particle size critically determine hardness, elastic modulus and wear resistance, with higher loadings and smaller particles conferring superior properties. Subsequent investigations confirmed that filler geometry further modulates mechanical behavior. Turssi et al.38 demonstrated that particle shape and dispersion significantly affect wear resistance and monomer conversion, impacting long-term stability. At the nanoscale, El-Safty et al.39 showed that incorporation of nanosized fillers enhances hardness and modulus relative to bulk-fill composites. Rodríguez et al.36 and Okamoto et al.40 further confirmed that well-dispersed small fillers improve microstructure and mechanical strength, whereas oversized or agglomerated particles reduce these benefits. These findings provide a mechanistic rationale for AFS formulations, which employ controlled filler architectures to optimize hardness, stiffness and wear resistance. Mechanical testing confirmed the observed compositional and morphological trends. AFS demonstrated the highest hardness and stiffness (HV = 51.9; HM = 396.74 N/mm²), indicating superior resistance to deformation and wear. FC showed intermediate values (HV = 42.7; HM = 183.05 N/mm²), whereas FIA was the softest and weakest (HV = 28.3; HM = 111.32 N/mm²), consistent with its higher organic matrix fraction. These findings highlight the strong interplay between filler composition, surface morphology and mechanical behavior in determining overall sealant performance. Literature reports hardness values for commercial sealants ranging within 19–99 HV4 and 21–75 HV,41 placing our measurements within expected ranges. In our study, indentation tests were performed using both traditional methods (yielding HV and HIT values) and instrumented indentation
testing, which additionally provided Martens hardness (HM) and work ratio (ηIT).
Martens hardness, based on the continuous recording of force and penetration depth, is less sensitive to the choice of test load and captures both elastic and plastic contributions to material deformation, offering a more reliable characterization of composite materials than conventional Vickers hardness.42 Work ratio (ηIT), in turn, quantifies the proportion of elastic to total work during indentation, thereby reflecting the material’s resilience and ability to recover from occlusal stresses.43 Notably, AFS exhibited the highest ηIT values among the tested sealants, confirming its superior elastic recovery and mechanical durability compared with FC and FIA.
Surface energy and wettability are critical determinants of adhesion in restorative dentistry, governing the ability of materials to form intimate contact with etched enamel and dentin. Experimental evidence demonstrates that decreased contact angles, e.g., from 58.8° ±4.1° to 49.1° ±5.7° upon exposure to acidic media, enhance wettability and adhesive potential.44 Materials with critical surface energy above 40 mJ/m², such as acrylic resins, generally exhibit strong adhesion, whereas BIS-GMA–based composites with lower energies show inferior bonding. High-energy substrates favor lower contact angles, promoting stronger adhesion through thermodynamically favorable interactions as described by the Young–Dupre equation.45 Optimal adhesion requires that the adhesive’s surface tension be lower than the substrate’s surface energy to achieve effective bonding.
Our measurements revealed moderate hydrophilicity for all tested sealants, with water contact angles of 57.7° (AFS), 63.9° (FC) and 60.5° (FIA). Angles below 90° indicate hydrophilicity, enhancing wetting and adhesion in moist environments.46 FC was the most hydrophilic, potentially improving its ability to seal pits and fissures in clinical conditions. Conversely, AFS and FIA, being relatively more hydrophobic, may resist moisture contamination during application, supporting long-term durability. Total surface free energy ranged from 50.7 mJ/m² (FC) to 55.1 mJ/m² (AFS), with the polar component highest for AFS (γSp = 14.2 mJ/m²), indicating superior potential for adhesion to enamel surfaces. Elevated surface energy and polar fraction facilitate intimate contact with hydrophilic tooth structures, improving wetting, retention and adhesive performance.
Fluoride release was evaluated in deionized water and artificial saliva at pH 4.5, 5.5, 7.0, and 7.5. AFS released the highest amounts under neutral and basic conditions (CFRI = 0.772 ppm at pH 7.5), whereas FIA released more in acidic conditions (pH 4.5) and deionized water. The initial burst release ranged within 8.2–17.9%, with maximum release times between 3–72 h and half-life values of 4–7 days to 2–3 weeks. Over 1,200 h, all materials acted as fluoride reservoirs, supporting remineralization and caries prevention. Differences in fluoride kinetics reflect variations in composition, filler type and excipients, consistent with previous studies.20, 47, 48 Tailored compositions, such as in AFS, provide prolonged fluoride availability under varying pH, enhancing anti-caries efficacy.
It has been reported that excipients in dentifrice can significantly influence the retention of fluoride in the oral cavity.49, 50 This concept may also extend to dental sealants, where the composition and excipients of the material could impact fluoride release and retention, thereby influencing their caries-preventive efficacy.
Clinical evidence corroborates these material characteristics. Systematic reviews report that resin-based sealants reduce caries incidence by 11–51% over 24 months, with benefits persisting up to 48 months.51 Hydrophilic sealants show superior short-term retention (odds ratio (OR) = 3.00 at 3 months; OR = 2.00 at 12 months), while long-term caries prevention is comparable to hydrophobic systems. Auto- and light-cured resin sealants achieve the highest long-term retention (up to 70% at 5 years), whereas glass ionomer and primer-modified sealants show lower retention (14–43% at 2–3 years). Performance is generally consistent across tooth types, though premolars may have slightly better outcomes. Comparisons of resin-based sealants and flowable composites indicate similar retention, though long-term data are limited.
In summary, the combination of higher filler content, optimized particle architecture, superior mechanical properties, favorable surface energy, and controlled fluoride release renders AFS a particularly promising sealant system. It exhibits the most favorable overall profile, with high mechanical strength, surface energy, polar fraction, and sustained fluoride release, supporting adhesion, wetting and resistance to occlusal forces. Moderate hydrophilicity ensures effective wetting in clinical conditions, while relative hydrophobicity during application reduces moisture sensitivity, promoting long-term retention.35 FC demonstrates intermediate performance, whereas FIA is mechanically weaker, but exhibits higher fluoride release under acidic conditions.
Strategies to enhance the performance of resin-based pit and fissure sealants focus on optimizing filler content and incorporating bioactive or appropriately selected nanofillers,52, 53 which can simultaneously improve mechanical, surface and antibacterial properties, as well as enhance physico-mechanical characteristics, thereby increasing their effectiveness in preventing dental caries.
Overall, these findings emphasize the importance of integrating physico-chemical, morphological, mechanical, and bioactive assessments when selecting pit and fissure sealants. AFS demonstrates superior clinical potential and longevity compared with FC and FIA, providing a mechanistic and clinical rationale for its preferential use in preventive dentistry.
Limitation
It is imperative that long-term in vitro studies are necessary to comprehensively evaluate the clinical performance of the tested sealants. Furthermore, it is imperative to take into account the feedback provided by clinicians to optimize the application procedures and its conditions. This will ensure that the prevention of caries is improved and that the sealant is retained in the long term.
Conclusions
The present study provides a comprehensive evaluation of the physico-chemical, morphological, mechanical, and fluoride-release properties of 3 commercially available dental sealants (AFS, FC, FIA), highlighting their direct impact on clinical performance. Among the tested materials, AFS exhibited the most favorable overall profile, combining high filler content, optimized particle architecture, superior mechanical strength, elevated surface energy, and sustained fluoride release, which together support robust adhesion, resistance to occlusal forces, and effective caries prevention. Its moderate hydrophilicity ensures efficient wetting and retention in moist clinical conditions, enhancing short-term performance, while its mechanical robustness and surface energetics, comparable to high-durability auto- and light-cured resin systems, indicate potential for superior long-term retention. AFS demonstrated superior effectiveness during evaluation, supporting its universal applicability in clinical practice, and its retention properties are comparable to flowable composites while providing additional advantages, such as improved hydrophilicity. Overall, these findings underscore the mechanistic and clinical rationale for the adhesive superiority, functional reliability, and long-term durability of AFS sealants, making them a particularly promising choice for preventive dentistry.