Abstract
Background. Polymethyl methacrylate (PMMA) is widely used as a denture base material despite its limitations, which include low transverse strength, impact resistance, surface hardness, and relatively high-water solubility and sorption. To enhance its mechanical and physical properties, PMMA has been modified by incorporating various metal powder fillers, such as aluminum and copper – despite their tendency to cause discoloration. These modifications aim to improve the overall quality and durability of dental prostheses.
Objectives. To evaluate the influence of incorporating microform propolis powder (known for its antifungal and antimicrobial properties and its rich composition of functional groups) into acrylic denture base material, and to assess its effect on selected physical and mechanical properties of the material.
Materials and methods. A total of 128 specimens were prepared to evaluate various mechanical properties. Four groups were tested: 1 control group containing acrylic resin without propolis, and 3 experimental groups with propolis powder added at concentrations of 1.0%, 2.0% and 3.0% by weight. Each group consisted of 8 specimens for each mechanical test. All specimens were cured using the conventional heat-curing method. The mechanical properties evaluated included transverse strength, impact strength, surface hardness, and surface roughness. The data were statistically analyzed using the IBM SPSS software.
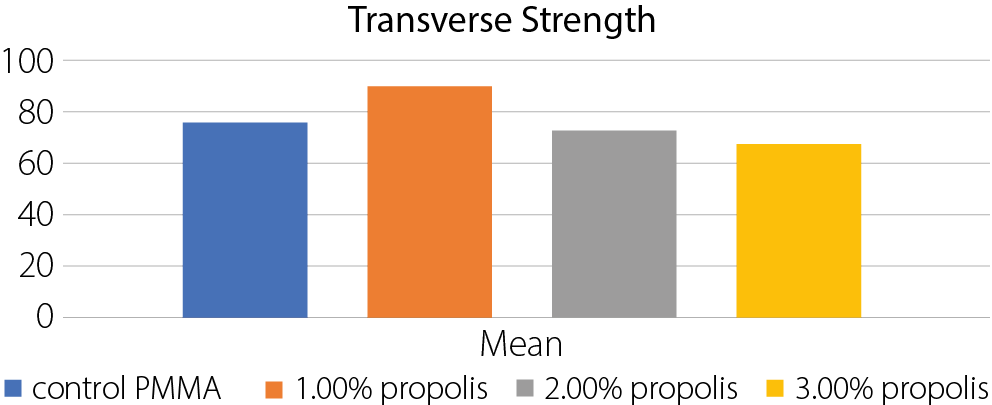
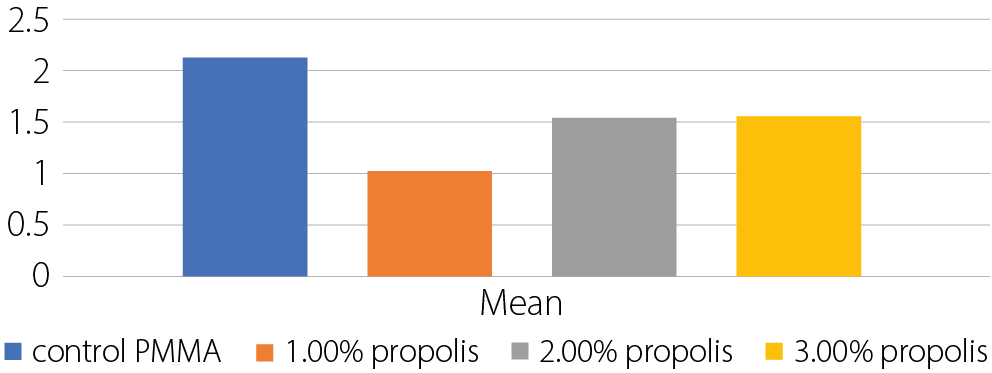
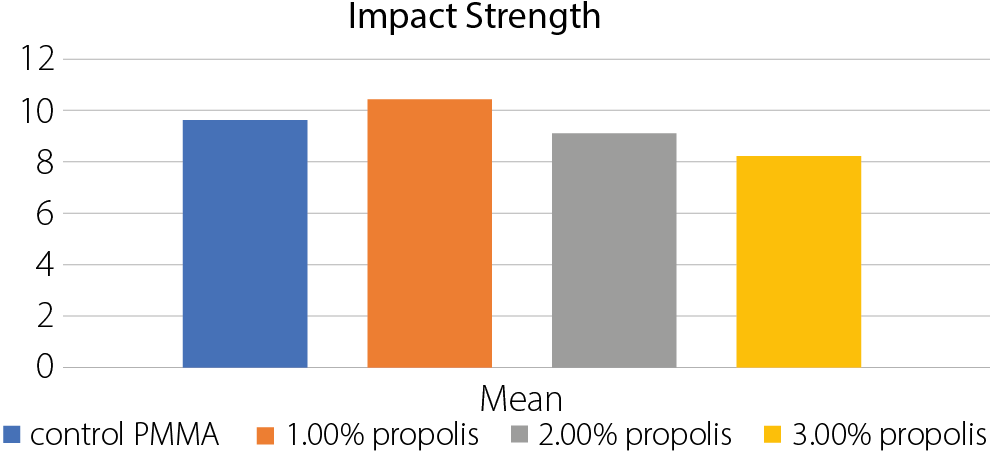
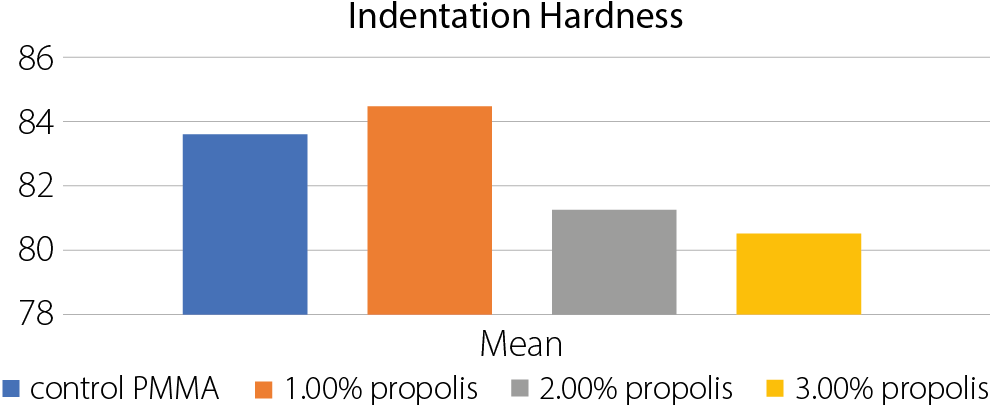
Results. The group with 1.0% propolis addition showed the highest mean values in all tested mechanical properties: transverse strength (90.50 N/mm²), impact strength (10.45 kJ/m²) and surface hardness (84.39). These values were significantly higher than those of the control group, with statistical analysis revealing highly significant differences between groups (p < 0.05) using ANOVA. Regarding surface roughness, the 1.0% propolis group also recorded the lowest mean value (1.03 µm), compared to the control group (2.14 µm), with all experimental groups showing significantly reduced roughness.
Conclusions. The incorporation of 1.0% microform propolis powder into PMMA denture base material significantly improved its mechanical and surface properties. These promising results suggest that further studies are warranted – either to explore additional properties or to test different propolis concentrations, potentially combined with coupling agents such as silane to enhance bonding and performance.
Key words: mechanical properties, polymethyl methacrylate, propolis
Introduction
Poly (methyl methacrylate) (PMMA) has been widely used for dental prostheses due to its advantageous properties. However, it fails to meet several mechanical demands, such as exhibiting low transverse strength, low impact strength, low surface hardness and high water solubility and sorption.1 Efforts to enhance PMMA include adding metal powder fillers like aluminum and copper, which improve properties but result in unacceptable color.2 Another approach involves reinforcing PMMA with fibers such as nylon, carbon and glass.3
Propolis, a natural resin collected by bees from plant exudates and shoots, has been used in traditional medicine since ancient times.4 In dentistry, propolis exhibits anti-inflammatory, antibacterial, antifungal, hemostatic, and tissue-rearrangement properties.5 Propolis, at a 76% concentration, added to silicone soft liners for hot-cured denture base material, effectively inhibits Candida albicans growth and enhances tensile strength.6 Research by Tobaldini-Valerio et al. demonstrated propolis’s antifungal impact on Candida albicans,7 preventing biofilm growth and eliminating mature biofilms. Candida albicans biofilms can reduce the mechanical properties of soft denture liners with heat-polymerized acrylic resin.8
Objectives
This study aims to investigate the effect of propolis micropowder on the mechanical and physical properties of PMMA denture base material at various concentrations, as well as its antifungal activity.
Materials and methods
The influence of propolis on heat-cured acrylic resin was analyzed by incorporating it into the acrylic powder at concentrations of 1%, 2% and 3%, while a propolis-free formulation served as the control. The additive, sieved to a fine 25-micron particle size, was mixed into the resin. Each concentration group consisted of 8 specimens, resulting in 32 samples per tested property. Altogether, 128 specimens were prepared and assessed to determine their mechanical characteristics.
Mold preparation
Molds were created using computer-measured plastic pattern blocks cut to precise dimensions. For transverse strength, hardness and surface roughness tests, specimens of 65 × 10 × 2.5 mm were manufactured. For impact strength tests, specimens of 80 × 10 × 4 mm were prepared.
The dental flask’s lower half was filled with prepared stone slurry mixed according to the ratio. Plastic specimens were submerged in the slurry, which was allowed to set for 1 h before being coated with separating media (alginate solution). The typical curing process followed, including investing, removing the plastic mold and packing. Acrylic’s power liquid (P/L) ratio was 2.5 g: 1.0 mL w/v per manufacturer’s instructions. The mixture was left at room temperature in a covered jar for 45 s after mixing with the monomer; then, the appropriate amount of polymer and propolis was added. Once the dough stage was reached, each flask was filled with resin. The 2 halves of the flask were then joined and placed in a press (HYDROFIX press; BEGO, Bremen, Germany) with slow pressure application to allow the dough to flow evenly throughout the mold space. The press was then turned off after 5 min. The flasks were clamped and placed in a curing bath at 70°C for 30 min, then raised to 100°C for 2 h. After cooling, the flasks were opened, and acrylic specimens were carefully extracted, finished and polished. Only roughness test specimens were left unpolished.
Mechanical testing
Transverse strength was tested using an Instron universal testing machine (WDW-200 E; Instron, Norwood, USA; Figure 1) Specimens were placed on a bending fixture with 2 parallel supports spaced 50 mm apart. The load was applied at a crosshead speed of 2 mm/min until fracture occurred.
Transverse strength (TS) was calculated using the following equation:
where L is the maximum load in newtons, W is the supporting width in mm, b is the width of the test specimen in mm, and h is the height of the test specimen in mm.
Impact strength was measured using the Charpy impact test for unnotched specimens. Specimens were struck by a free-swinging pendulum of 2 J, and impact energy was indicated by scale readings in joules. Impact strength (IS) was calculated using the equation:
where e is energy in joules, d is depth in mm and b is width in mm.
Surface hardness was measured using a Shore D durometer (Time Group Inc., Beijing, China), equipped with a 0.8 mm diameter spring-loaded indenter and a digital scale ranging from 0 to 100 units. The usual procedure is to press down swiftly and forcefully on the intender and then record the reading. Each specimen underwent 3 readings, 1 in the middle and 1 at each end, and the mean of the 3 readings was analyzed.
Specimens with dimensions of 65 × 10 × 2.5 ±0.1 mm were prepared. The effect of propolis particles on the surface microgeometry was assessed using a profilometer equipped with a sharp diamond-tipped stylus. This analyzer records the peaks and valleys that define the surface profile. The unpolished specimen was placed on a stable stage, and the test area was divided into 4 sections. The profilometer was moved across each section, and the average of the 4 readings was calculated.
Data analysis
Descriptive statistics, including mean and standard deviation (SD) values for each group, were processed using IBM SPSS v. 22 (IBM Corp., Armonk, USA). A one-way analysis of variance (ANOVA) test was conducted to determine the significance of propolis concentrations on the mechanical properties of heat-polymerized acrylic resin denture base material, with results from the least significant difference (LSD) analysis providing insights into the differences between the groups for each test.
Results
The following tables summarize the results of the 4 groups, defined by propolis concentration.
Transverse strength test
The highest mean transverse strength was observed in the group with 1% propolis (90.5 N/mm2), which decreased as the propolis percentage increased (Table 1, Table Figure 2). The ANOVA test results indicated highly significant differences between the groups (Table 2).
Surface roughness test
Experimental groups exhibited lower surface roughness values compared to the control group, which had a high value of 2.138 μm. The lowest surface roughness was found in the 1.0 wt% propolis samples (1.027 μm) (Table 3, Table Figure 3). The ANOVA test results revealed highly significant differences between the groups (Table 4).
Impact strength test
The highest mean impact strength was observed in
the 1.0 wt% propolis group (10.454 KJ/m2), with values decreasing but remaining close to the control group (Table 5, Table Figure 4). The ANOVA test results indicated highly significant differences between the groups (Table 6).
Indentation hardness test
Surface hardness increased in the 1.0 wt% propolis group (84.538) and decreased with higher propolis percentages (Table 7, Table Figure 5). The ANOVA test results showed highly significant differences between the groups (Table 8).
Least significant test
The results from the LSD analysis provide insights into the differences between the groups for each test. Here’s what it implies:
Transverse strength test
• Significant differences:
. Control PMMA vs 1% propolis;
. Control PMMA vs 3% propolis;
. 1% propolis vs 2% propolis;
. 1% propolis vs 3% propolis.
• Nonsignificant differences:
. Control PMMA vs 2% propolis;
. 2% propolis vs 3% propolis.
Implication: The addition of 1% propolis significantly increases the transverse strength compared to the control and other concentrations. However, 2% and 3% propolis
do not show significant differences from each other or
the control.
Surface roughness test
• Significant differences:
. Control PMMA vs all propolis concentrations (1%,
2%, 3%)
. 1% propolis vs 2% propolis
. 1% propolis vs 3% propolis
• Nonsignificant differences:
. 2% propolis vs 3% propolis
Implication: All concentrations of propolis significantly reduce surface roughness compared to the control. The 1% propolis concentration shows the most significant reduction, while 2% and 3% propolis do not differ significantly from each other.
Impact strength test
• Significant differences:
. Control PMMA vs 1% propolis;
. Control PMMA vs 3% propolis;
. 1% propolis vs 2% propolis;
. 1% propolis vs 3% propolis;
. 2% propolis vs 3% propolis.
• Nonsignificant differences:
. Control PMMA vs 2% propolis.
Implication: The 1% propolis concentration significantly increases impact strength compared to the control and other concentrations. The 3% propolis concentration significantly decreases impact strength compared to the control and other concentrations.
Indentation hardness test
• Significant differences:
. Control PMMA vs all propolis concentrations (1%, 2%, 3%);
. 1% propolis vs 2% propolis;
. 1% propolis vs 3% propolis.
• Nonsignificant differences:
. 2% propolis vs 3% propolis
Implication: The addition of 1% propolis significantly increases indentation hardness compared to the control and other concentrations. The 2% and 3% propolis concentrations significantly decrease indentation hardness compared to the control.
Overall implications
• 1% propolis: Generally, improves transverse strength, reduces surface roughness, increases impact strength, and increases indentation hardness.
• 2% propolis: Does not significantly differ from the control in most tests, except for surface roughness.
• 3% propolis: Generally, decreases transverse strength and impact strength, while reducing surface roughness and indentation hardness.
These results suggest that the optimal concentration of propolis for improving material properties varies depending on the specific property being tested.
Discussion
Propolis is a natural substance known for its wide range of biological activities, including antioxidant, antibacterial, antifungal, anti-inflammatory, antiviral, and anticancer effects.9, 10, 11 It is collected by bees from plant sources such as leaves and buds and mixed with pollen and wax to form a resinous material.12, 13 Chemically, propolis is complex, containing over 300 compounds such as phenolic acids, flavonoids, terpenes, amino acids, and various aromatic and fatty substances.14, 15, 16 This variability in composition can make standardization and quality control challenging.17, 18, 19
Typically, propolis consists of about 50% resin and vegetable balsam, 30% wax, 10% essential and aromatic oils, 5% pollen, and other minor components, including organic debris.20 In this study, propolis was chosen for its antifungal properties and its neutral color, which does not alter the appearance of the denture base material. Its phenolic-rich composition21 was incorporated into acrylic resin to explore its therapeutic potential and assess its influence on mechanical properties at different concentrations.22
The results showed that the highest transverse strength was achieved with 1% propolis. At higher concentrations, strength decreased compared to the control, with statistically significant differences confirmed with ANOVA. The reduction in strength at higher levels may be related to the wax content in propolis,20 which could interfere with the resin matrix. In contrast, the 1% concentration may benefit from aromatic functional groups that enhance bonding within the polymer. The absence of coupling agents like silane, which typically improve adhesion, may also have contributed to the observed trends.
Surface roughness was lowest in the 1% propolis group, and all tested concentrations showed values below that of the control. These differences were statistically significant. A smoother surface is advantageous as it reduces bacterial adhesion and the risk of oral infections, consistent with findings by de Foggi et al.23 This application of propolis appears to be novel, and the presence of wax and resinous components may have influenced the surface characteristics.
Propolis also offers several pharmacological benefits, including wound healing, anesthetic, hemostatic, anticariogenic, anti-inflammatory, and antibiotic effects,24, 25, 26 and is considered non-cytotoxic to humans.21 The increase in impact strength observed at 1% propolis suggests good dispersion and compatibility with the resin. Its plasticizing effect may contribute to improved flexibility, and the presence of functional groups could facilitate bonding with the polymer matrix.
Surface hardness increased slightly at 1% propolis but declined at higher concentrations. These differences were also statistically significant. The decrease in hardness at higher levels may be due to the absence of hard fillers in propolis and the influence of phenolic and antioxidant compounds, which can affect the structural integrity of the polymer.27
A study by Al-Khalifa et al.28 reported similar findings, where 2.5% propolis in PMMA reduced C. albicans counts and increased surface roughness, while higher concentrations led to decreased hardness. These results support the current findings and highlight the potential of propolis as a bioactive additive. Incorporating 1% propolis into heat-cured acrylic resin appears to enhance transverse and impact strength, improve surface hardness, and reduce roughness, suggesting potential benefits for the performance and longevity of denture base materials.
Limitations
One challenge we encountered was related to the color of the material. Increasing the additive concentration beyond the level we used could affect the esthetic quality, making it less similar to the natural color of gum tissue. For this reason, we limited the concentration to 3% to maintain acceptable aesthetic properties. Additionally, since the material contains several active chemical groups, it is not yet clear which specific reactions are responsible for the results we observed. Further investigations are needed to identify the primary chemical reactions responsible for the observed effects.
Conclusions
This study highlights the significant potential of propolis as a new source of bioactive compounds and its ability to improve the mechanical properties of heat-cured acrylic resin denture bases. Incorporating 1% propolis powder may enhance the transverse and impact strength, as well as the hardness, while decreasing surface roughness. These improvements suggest that propolis incorporation could enhance
the stability and shelf-life of commercial acrylic materials.
Use of AI and AI-assisted technologies
Not applicable.